AOD-9604 + CJC-1295 + Ipamorelin (12 mg Vial) Dosage Protocol
Quickstart Highlights
This synergistic blend combines three peptides targeting body composition: AOD-9604 (hGH fragment 177–191) promotes lipolysis without raising IGF-1[1]; CJC-1295 (GHRH analog) produces sustained GH and IGF-1 elevations[2]; and Ipamorelin (ghrelin mimetic) selectively stimulates GH secretion without increasing cortisol or ACTH[3]. Together, these peptides may support lean mass gains and fat reduction[4].
- Vial contents: 6 mg AOD-9604 + 3 mg CJC-1295 + 3 mg Ipamorelin (12 mg total).
- Reconstitute: Add 3.0 mL bacteriostatic water → 4 mg/mL total (2 mg/mL AOD, 1 mg/mL CJC, 1 mg/mL Ip).
- Typical daily range: 0.1–0.2 mL once daily (200–400 mcg AOD / 100–200 mcg CJC / 100–200 mcg Ip).
- Easy measuring: At 4 mg/mL total, 1 unit = 0.01 mL = 40 mcg total (20 mcg AOD + 10 mcg CJC + 10 mcg Ip).
- Storage: Lyophilized: freeze at −20 °C (−4 °F); after reconstitution, refrigerate at 2–8 °C (35.6–46.4 °F); avoid freeze–thaw cycles.

Dosing & Reconstitution Guide
Educational guide for reconstitution and daily dosing
Standard / Gradual Approach (3 mL reconstitution)
Concentration after reconstitution: 2.0 mg/mL AOD-9604 | 1.0 mg/mL CJC-1295 | 1.0 mg/mL Ipamorelin
| Week | Daily Dose | Units (mL) |
|---|---|---|
| Week 1 | 200 mcg AOD / 100 mcg CJC / 100 mcg Ip | 10 units (0.10 mL) |
| Week 2 | 300 mcg AOD / 150 mcg CJC / 150 mcg Ip | 15 units (0.15 mL) |
| Weeks 3–12 | 400 mcg AOD / 200 mcg CJC / 200 mcg Ip | 20 units (0.20 mL) |
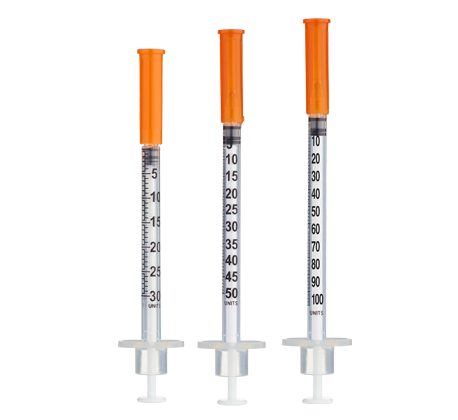
Frequency: Inject once daily subcutaneously, preferably in the morning or before bed on an empty stomach. For ≤10‑unit (≤0.10 mL) administrations during titration, consider 30‑ or 50‑unit insulin syringes for improved readability.
Reconstitution Steps
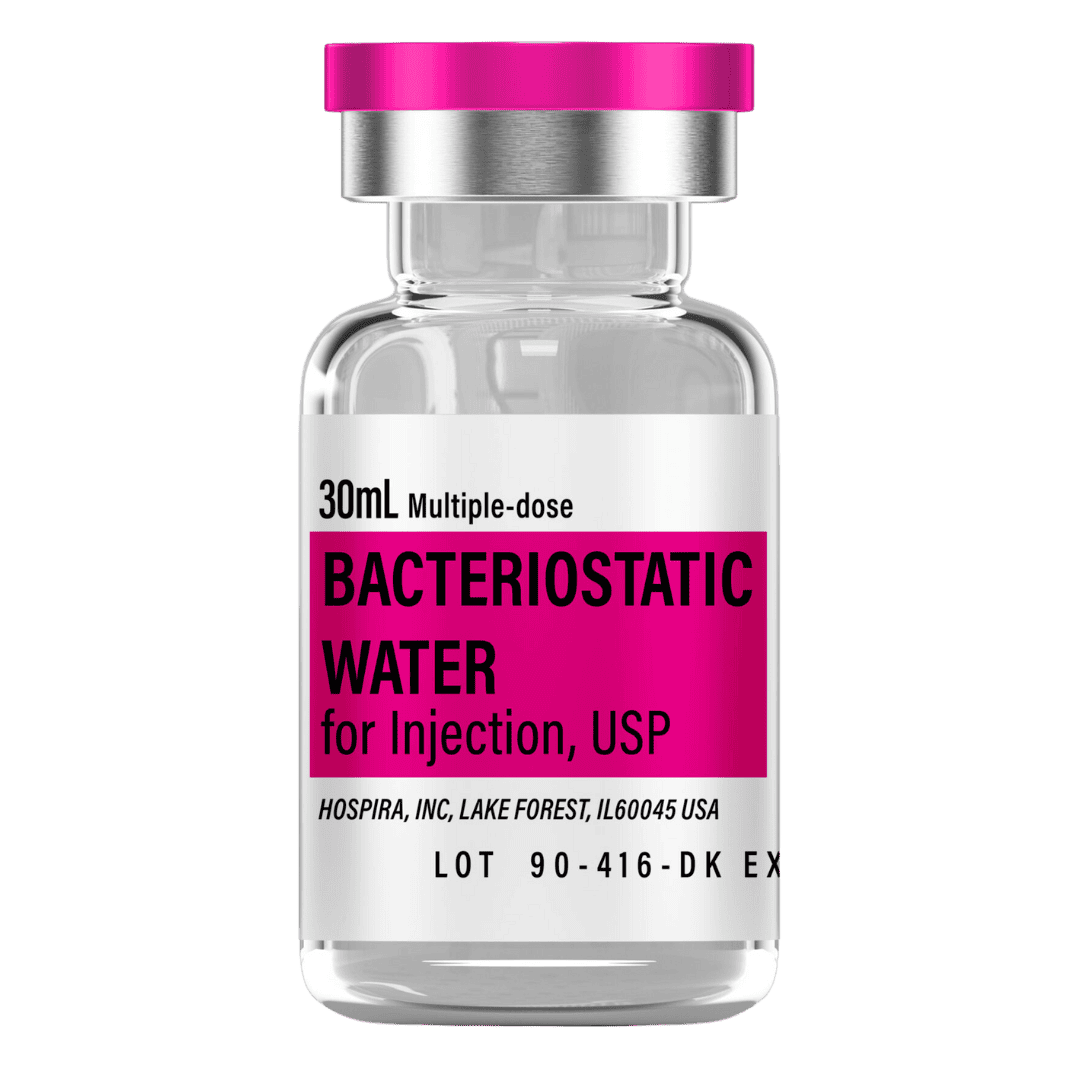
- Draw 3.0 mL bacteriostatic water with a sterile syringe.
- Inject slowly down the vial wall; avoid foaming.
- Gently swirl/roll until dissolved (do not shake).
- Label and refrigerate at 2–8 °C (35.6–46.4 °F), protected from light.
Syringe Math Reference
Using a U-100 insulin syringe (100 units = 1 mL):
- 1 unit (0.01 mL) = 20 mcg AOD + 10 mcg CJC + 10 mcg Ip
- 10 units (0.10 mL) = 200 mcg AOD + 100 mcg CJC + 100 mcg Ip
- 15 units (0.15 mL) = 300 mcg AOD + 150 mcg CJC + 150 mcg Ip
- 20 units (0.20 mL) = 400 mcg AOD + 200 mcg CJC + 200 mcg Ip
Supplies Needed
Plan based on an 8–16 week daily protocol with gradual titration (maintenance dose: 0.20 mL/day).
-
Peptide Blend Vials (12 mg each):
- 8 weeks (~11.2 mL total): 4 vials
- 12 weeks (~16.8 mL total): 6 vials
- 16 weeks (~22.4 mL total): 8 vials
-
Insulin Syringes (U‑100):
- Per week: 7 syringes (1/day)
- 8 weeks: 56 syringes
- 12 weeks: 84 syringes
- 16 weeks: 112 syringes
-
Bacteriostatic Water (10 mL bottles): Use 3.0 mL per vial for reconstitution.
- 8 weeks (4 vials): 12 mL → 2 × 10 mL bottles
- 12 weeks (6 vials): 18 mL → 2 × 10 mL bottles
- 16 weeks (8 vials): 24 mL → 3 × 10 mL bottles
-
Alcohol Swabs: One for the vial stopper + one for the injection site each day.
- Per week: 14 swabs (2/day)
- 8 weeks: 112 swabs → recommend 2 × 100‑count boxes
- 12 weeks: 168 swabs → recommend 2 × 100‑count boxes
- 16 weeks: 224 swabs → recommend 3 × 100‑count boxes
Protocol Overview
Concise summary of the once‑daily regimen.
- Goal: Support body composition improvements through combined lipolytic and GH-stimulating mechanisms[4][5].
- Schedule: Daily subcutaneous injections for 8–12 weeks (extend to 16 weeks if desired).
- Dose Range: 200–400 mcg AOD / 100–200 mcg CJC / 100–200 mcg Ip daily with gradual titration.
- Reconstitution: 3.0 mL per 12 mg vial (4 mg/mL total concentration).
- Storage: Lyophilized frozen; reconstituted refrigerated; avoid repeated freeze–thaw.
Dosing Protocol
Suggested daily titration approach.
- Start: 0.10 mL daily (200 mcg AOD / 100 mcg CJC / 100 mcg Ip); increase by ~0.05 mL weekly as tolerated.
- Target: 0.20 mL daily (400 mcg AOD / 200 mcg CJC / 200 mcg Ip) by Week 3.
- Frequency: Once per day (subcutaneous).
- Cycle Length: 8–12 weeks; optional extension to 16 weeks.
- Timing: Morning or before bed on an empty stomach; rotate injection sites.
Storage Instructions
Proper storage preserves peptide quality.
- Lyophilized: Store at −20 °C (−4 °F) in dry, dark conditions; minimize moisture exposure.
- Reconstituted: Refrigerate at 2–8 °C (35.6–46.4 °F); use within 28 days per CDC/USP guidelines[6].
- Allow vials to reach room temperature before opening to reduce condensation uptake.
Important Notes
Practical considerations for consistency and safety.
- Use new sterile insulin syringes for each injection; dispose in a sharps container.
- Rotate injection sites (abdomen, thighs, upper arms) to reduce local irritation.
- Inject slowly; wait a few seconds before withdrawing the needle.
- Document daily dose and site rotation to maintain consistency.
- Best administered on an empty stomach (fasting) for optimal GH release.
How This Works
This blend combines three complementary peptides with distinct mechanisms:
- AOD-9604 is a modified C-terminal fragment of human growth hormone (amino acids 177–191) that retains the lipolytic and anti-lipogenic properties of GH without stimulating IGF-1 or affecting glucose metabolism[1][7].
- CJC-1295 is a long-acting GHRH analog that produces sustained elevations in growth hormone and IGF-1 levels[2], supporting anabolic processes and fat metabolism.
- Ipamorelin is a selective ghrelin mimetic that stimulates pulsatile GH secretion without significantly increasing ACTH or cortisol[3], offering a cleaner GH release profile.
Together, these peptides work synergistically to enhance endogenous GH pulsatility and promote favorable changes in body composition similar to exogenous GH therapy[4][5].
Potential Benefits & Side Effects
Observations from preclinical and clinical literature.
Potential Benefits
- Supports reduction in fat mass and enhancement of lipolysis[1][5].
- May promote lean mass gains through sustained GH and IGF-1 elevation[2][4].
- Selective GH release without significant cortisol or prolactin increases[3].
- AOD-9604 shows a placebo-like safety profile in human studies[7][8].
Possible Side Effects
- Mild injection-site reactions (redness, itching) with subcutaneous administration.
- Transient flushing or warmth following injection.
- Occasional headache or lightheadedness during initial use.
- Water retention (typically mild) with sustained GH elevation.
Lifestyle Factors
Complementary strategies for best outcomes.
- Pair with a balanced, protein-forward diet tailored to energy needs.
- Combine resistance training and aerobic activity to reinforce metabolic adaptations.
- Prioritize quality sleep (7–9 hours) to maximize endogenous GH release.
- Manage stress to optimize hormonal balance and recovery.
- Stay hydrated and maintain consistent meal timing.
Injection Technique
General subcutaneous guidance from clinical best-practice resources[9].
- Clean the vial stopper and skin with alcohol; allow to dry.
- Pinch a skinfold; insert the needle at 45–90° into subcutaneous tissue[10][11].
- Do not aspirate for subcutaneous injections; inject slowly and steadily[10].
- Rotate sites systematically (abdomen, thighs, upper arms) to avoid lipohypertrophy[12].
- Dispose of used syringes in an approved sharps container; follow local disposal regulations.
Recommended Source
We recommend Pure Lab Peptides for high-purity AOD-9604 + CJC-1295 + Ipamorelin Blend (12 mg).
Why Pure Lab Peptides?
- High-purity, third-party-tested lots with batch COAs.
- Consistent, ISO-aligned handling and documentation.
- Reliable fulfillment to maintain cold-chain integrity.
Important Note
This content is intended for therapeutic educational purposes only and does not constitute medical advice, diagnosis, or treatment.
References
-
Journal of Endocrinology & Metabolism (2014)
— Safety and metabolism of AOD9604: novel nutraceutical ingredient for improved metabolic health -
Journal of Clinical Endocrinology & Metabolism (PubMed)
— Prolonged stimulation of GH and IGF-I secretion by CJC-1295 in healthy adults -
European Journal of Endocrinology (PubMed)
— Ipamorelin: the first selective growth hormone secretagogue -
Translational Andrology and Urology (PMC)
— Role of growth hormone secretagogues in body composition management -
Endocrinology (OUP)
— hGH and AOD-9604: lipid metabolism and beta-adrenergic pathway insights -
CDC
— Preventing unsafe injection practices: multi-dose vial handling guidelines -
Journal of Endocrinology & Metabolism (2013)
— Safety and tolerability of the hexadecapeptide AOD9604 in humans -
Obesity Pharmacotherapy Review (PMC)
— AOD-9604 clinical trial overview and weight loss outcomes -
Subcutaneous Drug Injection Review (PMC)
— Pharmacologic considerations of the subcutaneous route -
CDC
— Vaccine administration: subcutaneous route (angle/site guidance) -
MedlinePlus
— Subcutaneous injections: patient instructions and technique -
NCBI Bookshelf
— Best practices for injection: asepsis, preparation, and administration -
Central & Peripheral Anti-Obesity Targets (PMC)
— AOD-9604 RCT summary and mechanistic overview -
PubMed
— Metabolic studies of AOD-9604 in obese rodents (oral dosing, fat oxidation) -
Pure Lab Peptides
— AOD-9604 + CJC-1295 + Ipamorelin Blend product page (quality and batch documentation)