IGF-1 LR3 (1 mg Vial) Dosage Protocol
Quickstart Highlights
IGF-1 LR3 (Long R3 Insulin-like Growth Factor-1) is a modified analog of human IGF-1 with significantly extended half-life, studied for its anabolic and metabolic effects[1]. This synthetic variant exhibits reduced binding to IGF binding proteins, allowing enhanced bioavailability and systemic activity[2]. This educational protocol presents a once-daily subcutaneous approach with conservative titration for research applications.
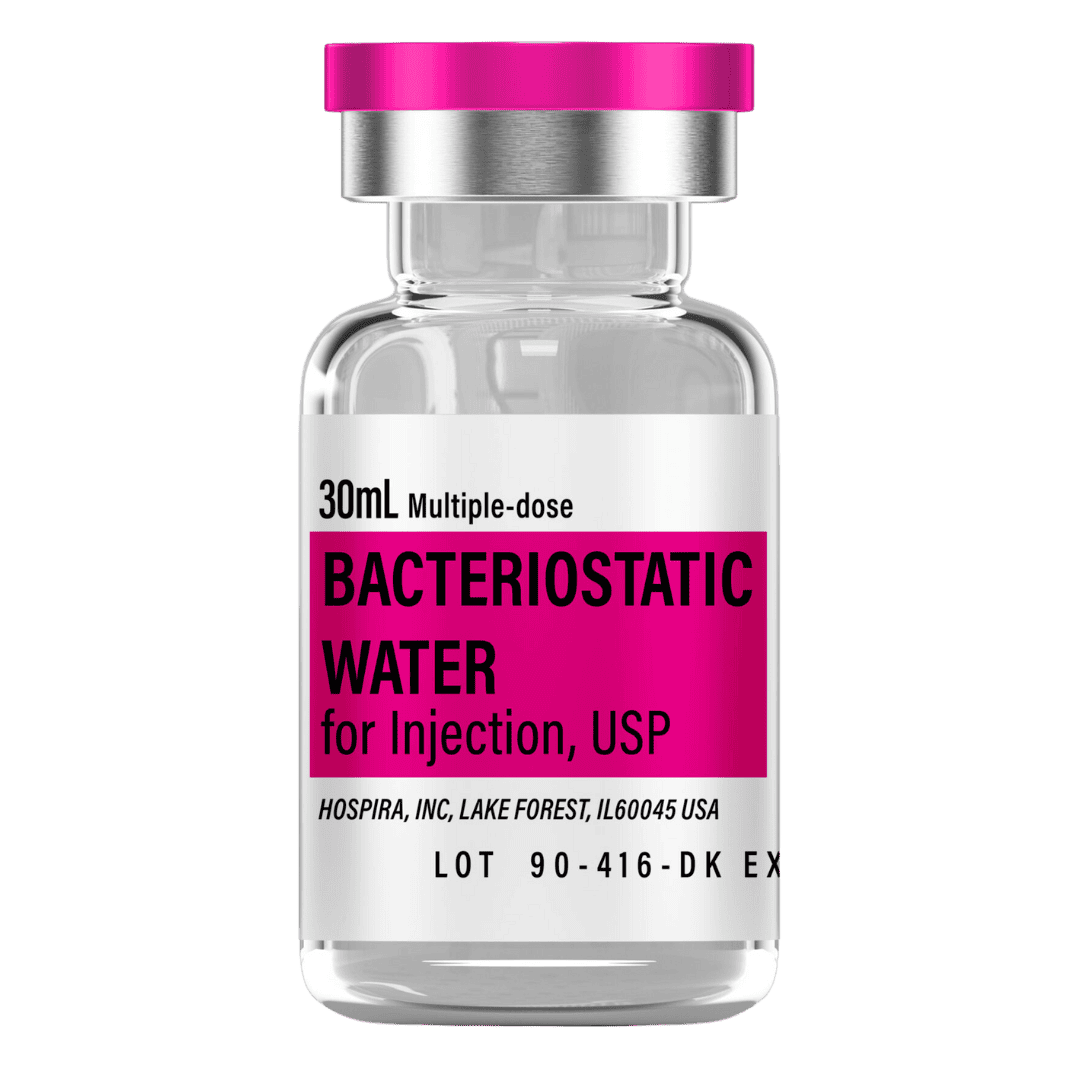
- Reconstitute: Add 3.0 mL bacteriostatic water → ~0.333 mg/mL concentration (333 mcg/mL).
- Typical daily range: 20–50 mcg once daily subcutaneously (gradual titration recommended).
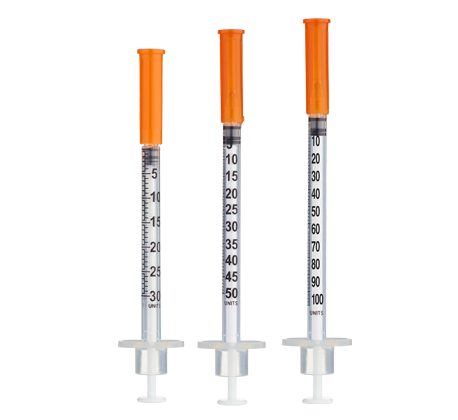
- Easy measuring: At 0.333 mg/mL, 1 unit = 0.01 mL ≈ 3.33 mcg on a U-100 insulin syringe.
- Storage: Lyophilized: freeze at −20 °C (−4 °F) for up to 12 months; after reconstitution, refrigerate at 2–8 °C (35.6–46.4 °F) and use within 30 days; avoid repeated freeze–thaw cycles.

Dosing & Reconstitution Guide
Educational guide for reconstitution and daily dosing
Standard / Gradual Approach (3 mL = ~0.333 mg/mL)
| Week | Daily Dose (mcg) | Units (per injection) (mL) |
|---|---|---|
| Weeks 1–2 | 20 mcg (0.02 mg) | 6 units (0.06 mL) |
| Weeks 3–4 | 40 mcg (0.04 mg) | 12 units (0.12 mL) |
| Weeks 5–8 | 50 mcg (0.05 mg) | 15 units (0.15 mL) |
Frequency: Inject once daily subcutaneously, typically in the morning or post-workout with food intake to mitigate insulin-like effects on blood glucose[3]. This schedule uses the maximum practical dilution (3.0 mL) for clear measurements. For ≤10-unit (≤0.10 mL) administrations, consider 30- or 50-unit insulin syringes for improved readability.
Reconstitution Steps
- Draw 3.0 mL bacteriostatic water with a sterile syringe.
- Inject slowly down the vial wall to avoid foaming; do not shake.
- Gently swirl or roll until the lyophilized powder dissolves completely into a clear solution.
- Label the vial with reconstitution date and refrigerate at 2–8 °C (35.6–46.4 °F), protected from light.
- Use within 30 days of reconstitution; for longer storage, prepare aliquots and freeze at −20 °C (−4 °F) for up to 3–6 months[4].
Supplies Needed
Plan based on an 8–16 week daily protocol with gradual titration.
-
Peptide Vials (IGF-1 LR3, 1 mg each):
- 8 weeks ≈ 3 vials (~2.24 mg total needed)
- 12 weeks ≈ 4 vials (~3.64 mg total needed)
- 16 weeks ≈ 6 vials (~5.04 mg total needed)
-
Insulin Syringes (U-100, 0.5 mL or 1 mL):
- Per week: 7 syringes (1/day)
- 8 weeks: 56 syringes
- 12 weeks: 84 syringes
- 16 weeks: 112 syringes
-
Bacteriostatic Water (10 mL bottles): Use 3.0 mL per vial for reconstitution.
- 8 weeks (3 vials): 9 mL → 1 × 10 mL bottle
- 12 weeks (4 vials): 12 mL → 2 × 10 mL bottles
- 16 weeks (6 vials): 18 mL → 2 × 10 mL bottles
-
Alcohol Swabs: One for the vial stopper + one for the injection site each day.
- Per week: 14 swabs (2/day)
- 8 weeks: 112 swabs → recommend 2 × 100-count boxes
- 12 weeks: 168 swabs → recommend 2 × 100-count boxes
- 16 weeks: 224 swabs → recommend 3 × 100-count boxes
- Sharps Container: For safe disposal of used needles and syringes[5].
- Fast-acting carbohydrate source: Glucose tablets or juice on hand during cycle to address potential hypoglycemia symptoms, especially during dose titration[6].
Protocol Overview
Concise summary of the once-daily subcutaneous regimen.
- Goal: Support anabolic processes and metabolic function through enhanced IGF-1 activity with extended bioavailability[1].
- Schedule: Daily subcutaneous injections for 8 weeks (standard cycle); may extend to 12 weeks with appropriate off-periods.
- Dose Range: 20–50 mcg daily with gradual titration; conservative protocols remain at ≤50 mcg/day.
- Reconstitution: 3.0 mL per 1 mg vial (~0.333 mg/mL or 333 mcg/mL) for precise unit measurements.
- Storage: Lyophilized powder frozen at −20 °C (−4 °F); reconstituted solution refrigerated at 2–8 °C (35.6–46.4 °F); avoid repeated freeze–thaw cycles.
- Cycling: Common approach is 8 weeks on, 4–8 weeks off to prevent receptor desensitization[7].
Dosing Protocol
Suggested daily titration approach for tolerance assessment.
- Start (Weeks 1–2): 20 mcg daily to assess tolerance, particularly regarding blood glucose effects[6].
- Titrate (Weeks 3–4): Increase to 40 mcg daily if Week 1–2 well-tolerated with no significant hypoglycemic symptoms.
- Maintain (Weeks 5–8): Hold at 50 mcg daily; this is considered the conservative upper end for most research protocols[2].
- Frequency: Once per day subcutaneously; timing often aligned with meals (morning or post-workout) to manage insulin-like effects.
- Cycle Length: 8 weeks is standard; 12-week protocols exist but may show diminished returns beyond Week 6–8[7].
- Site Rotation: Rotate injection sites systematically (abdomen, thighs, upper arms) to prevent local irritation or lipohypertrophy[8].
Storage Instructions
Proper storage is critical to maintain peptide stability and potency.
- Lyophilized (unopened): Store at −20 °C (−4 °F) or colder (−80 °C optimal) in dry, dark conditions for up to 12 months[4]; short-term storage at 2–8 °C (35.6–46.4 °F) for several months is acceptable.
- Reconstituted solution: Refrigerate at 2–8 °C (35.6–46.4 °F) immediately after mixing; use within 30 days for optimal potency[9].
- Extended storage of reconstituted solution: For storage beyond 30 days, prepare sterile aliquots and freeze at −20 °C (−4 °F); aliquots remain stable for 3–6 months[4]. Never refreeze a thawed vial.
- Handling: Allow frozen vials to reach room temperature before opening to minimize condensation; always inspect solution for clarity (discard if cloudy or contains particles)[10].
- Protection: Keep all vials protected from light and maintain cold chain during storage.
Important Notes
Practical considerations for consistency, safety, and optimal outcomes.
- Sterile technique: Always use new, sterile insulin syringes for each injection; dispose immediately in a sharps container[5]. Follow “one needle, one syringe, only one time” practice.
- Site rotation: Systematically rotate injection sites between abdomen (at least 2 inches from navel), outer thighs, and upper arms to reduce local irritation and prevent tissue hardening[8].
- Injection technique: Inject slowly and steadily; wait a few seconds before withdrawing the needle to prevent solution leakage.
- Hypoglycemia awareness: Be vigilant for signs of low blood sugar (shakiness, dizziness, sweating) especially during dose escalation; have fast-acting carbohydrates readily available[6].
- Timing with meals: Administer with or shortly after food intake to mitigate insulin-like effects on blood glucose[3].
- Documentation: Keep a daily log of dose, injection site, and any observed effects to maintain protocol consistency and identify tolerance patterns.
- Never exceed conservative dosing: Doses above 50–60 mcg/day lack robust clinical research support and may substantially increase adverse effect risk[2].
How This Works
IGF-1 LR3 is a synthetic analog of human insulin-like growth factor-1 engineered with an N-terminal extension (13 amino acids) and a glutamic acid substitution at position 3, resulting in significantly reduced binding affinity to IGF binding proteins[1]. This modification extends the peptide’s half-life from minutes (native IGF-1) to several hours and enhances systemic bioavailability[2].
The extended circulation time allows for once-daily administration protocols in research settings. Unlike native IGF-1, which requires frequent dosing, IGF-1 LR3 maintains more stable plasma levels throughout the day[11]. The peptide exhibits anabolic and metabolic activities through IGF-1 receptor binding, though it has never been approved for therapeutic human use and remains confined to research applications[12].
Studies examining IGF-1 and its analogs demonstrate effects on cellular growth, protein synthesis, and metabolic regulation. The insulin-like properties of IGF-1 LR3 necessitate careful attention to blood glucose management, particularly during initial dosing and titration phases[6].
Potential Benefits & Side Effects
Observations from preclinical and clinical literature on IGF-1 and its analogs.
Potential Research Observations:
- Enhanced anabolic signaling and protein synthesis in target tissues[1].
- Extended bioavailability compared to native IGF-1 due to reduced binding protein interaction[2].
- Metabolic effects through IGF-1 receptor pathways.
Important Safety Considerations:
- Hypoglycemia risk: The most significant concern with IGF-1 LR3 is its insulin-like effect on blood glucose; symptoms may include shakiness, confusion, sweating, or dizziness[6]. Always administer with food and monitor for glucose-related symptoms.
- No FDA approval: IGF-1 LR3 has never received regulatory approval for human therapeutic use and is restricted to research applications[12].
- Injection site reactions: Mild local irritation, redness, or discomfort may occur; proper technique and site rotation minimize these effects[8].
- Dose-dependent effects: Safety concerns increase substantially at doses exceeding 50–60 mcg/day; conservative dosing protocols are strongly recommended[2].
- Receptor desensitization: Continuous use beyond 6–8 weeks may lead to diminished response; cycling protocols (e.g., 8 weeks on, 4–8 weeks off) are commonly employed[7].
Lifestyle Factors
Complementary strategies to support research protocol outcomes.
- Nutrition: Maintain adequate protein intake (1.6–2.2 g/kg body weight) to support anabolic processes; ensure regular meal timing to manage blood glucose stability.
- Training: Combine resistance training protocols with appropriate recovery periods; IGF-1 signaling pathways are activated by mechanical loading.
- Sleep & Recovery: Prioritize 7–9 hours of quality sleep nightly to optimize endogenous growth factor production and tissue repair processes.
- Hydration: Maintain consistent hydration status to support metabolic function and peptide distribution.
- Glucose monitoring: Consider periodic blood glucose checks during dose titration, especially if experiencing any hypoglycemic symptoms.
Injection Technique
Subcutaneous injection guidance based on clinical best practices[13][14].
Preparation:
- Wash hands thoroughly with soap and water.
- Clean the vial stopper with an alcohol swab; allow to air dry completely.
- Ensure the reconstituted solution is clear with no visible particles; discard if cloudy[10].
- Draw the calculated dose into a sterile insulin syringe (29–31 gauge, ½ inch or 5/16 inch needle).
- Remove air bubbles by gently tapping the syringe and pushing plunger until solution is at the correct measurement.
Injection Procedure:
- Site selection: Choose areas with adequate subcutaneous fat—abdomen (2+ inches from navel), outer thighs, upper arms, or upper outer buttocks[8].
- Clean injection site: Swab with alcohol and allow to dry completely (do not fan or blow on site).
- Pinch technique: Gently pinch a fold of skin between thumb and forefinger to ensure subcutaneous placement[13].
- Needle insertion: Insert needle at 45–90° angle into the pinched skin fold (45° for thin individuals; up to 90° for those with more subcutaneous fat)[13][14].
- Injection: Do not aspirate for subcutaneous injections[13]; slowly depress plunger to inject peptide solution over 2–3 seconds.
- Withdrawal: Remove needle at the same angle it entered; apply gentle pressure with a clean cotton ball or swab (do not rub vigorously).
- Disposal: Immediately place used syringe/needle in a sharps container without recapping[5].
Site Rotation Strategy:
- Maintain a rotation pattern across multiple sites (e.g., left abdomen → right abdomen → left thigh → right thigh).
- Never inject into the exact same spot within 1–2 weeks; allow tissue recovery time.
- Avoid areas with scar tissue, moles, or skin irritation.
- Keep a simple rotation log to track injection sites and maintain systematic coverage.
Recommended Source
We recommend Pure Lab Peptides for high-purity IGF-1 LR3 (1 mg).
Why Pure Lab Peptides?
- Third-party testing: All lots undergo independent verification with batch-specific Certificates of Analysis (COAs) available for review.
- Purity standards: Products meet or exceed 98% purity specifications with proper characterization.
- Handling protocols: ISO-aligned cold chain management from manufacture through fulfillment.
- Documentation: Comprehensive product documentation, storage guidelines, and reconstitution instructions provided.
- Reliable fulfillment: Consistent shipping practices maintain product integrity during transit.
Important Note
This content is intended for therapeutic educational purposes only and does not constitute medical advice, diagnosis, or treatment. IGF-1 LR3 is for research use only and has not been approved by the FDA for human therapeutic applications. Always consult qualified healthcare professionals before considering any research peptide protocols. This information is provided for educational purposes to support informed research decisions.
References
-
Biomolecules (MDPI)
— Insulin-Like Growth Factor-1 (IGF-1) and Its Monitoring in Medical Diagnostic and in Sports -
Dopinglinkki (FINCIS Anti-Doping Authority)
— Insulin-like growth factor 1 (IGF-1) and long chain IGF (LR3IGF-1): Medical information and safety overview -
Mayo Clinic / IBM Micromedex
— Mecasermin (Subcutaneous route): Proper Use and Handling – guidance on IGF-1 administration timing with meals -
Cell Sciences (Research Reagents)
— Recombinant Human LR3 IGF-1: Product technical datasheet including storage and stability specifications -
Centers for Disease Control and Prevention (CDC)
— Preventing Unsafe Injection Practices: One Needle, One Syringe, Only One Time -
Drugs.com
— Increlex (mecasermin) Dosage Guide: Clinical dosing and hypoglycemia management for IGF-1 therapy -
AnabolicMinds Community Research Forum
— Storage and cycle considerations for IGF-1 LR3: Practical protocol discussions -
Immunize.org (Immunization Action Coalition)
— How to Administer Subcutaneous Vaccine Injections: Site selection and rotation guidance -
ReliaTech GmbH
— Human IGF-1 LR3 Protein Technical Specifications: Reconstitution and storage protocols -
Mayo Clinic
— Mecasermin (subcutaneous route): Side effects and proper handling – solution inspection guidelines -
Frontiers in Bioengineering & Biotechnology
— Insulin-Like Growth Factor-1: A Promising Therapeutic Target for Peripheral Nerve Injury -
Drug Testing and Analysis (PubMed)
— Detection of LongR3-IGF-I, Des(1-3)-IGF-I, and R3-IGF-I using immunopurification and high resolution mass spectrometry for antidoping purposes -
Centers for Disease Control and Prevention (CDC)
— Chapter 6: Vaccine Administration (Pink Book): Comprehensive subcutaneous injection technique guidance -
NCBI Bookshelf
— 5.6 Administering Subcutaneous Medications: Clinical best practices for subcutaneous injection technique -
Pure Lab Peptides
— IGF-1 LR3 (1 mg) Product Page: Supplier specifications, purity documentation, and COA access