Tirzepatide (10 mg Vial) Dosage Protocol
Quickstart Highlights
Tirzepatide is a 39–amino acid dual incretin receptor agonist that activates both GLP‑1 and GIP receptors, enhancing glucose‑dependent insulin secretion, suppressing glucagon, slowing gastric emptying, and reducing appetite[1][2]. Its ~5‑day half‑life allows convenient once‑weekly subcutaneous dosing[1]. Clinical trials demonstrate superior glycemic control and weight reduction compared to selective GLP‑1 agonists[3][4].
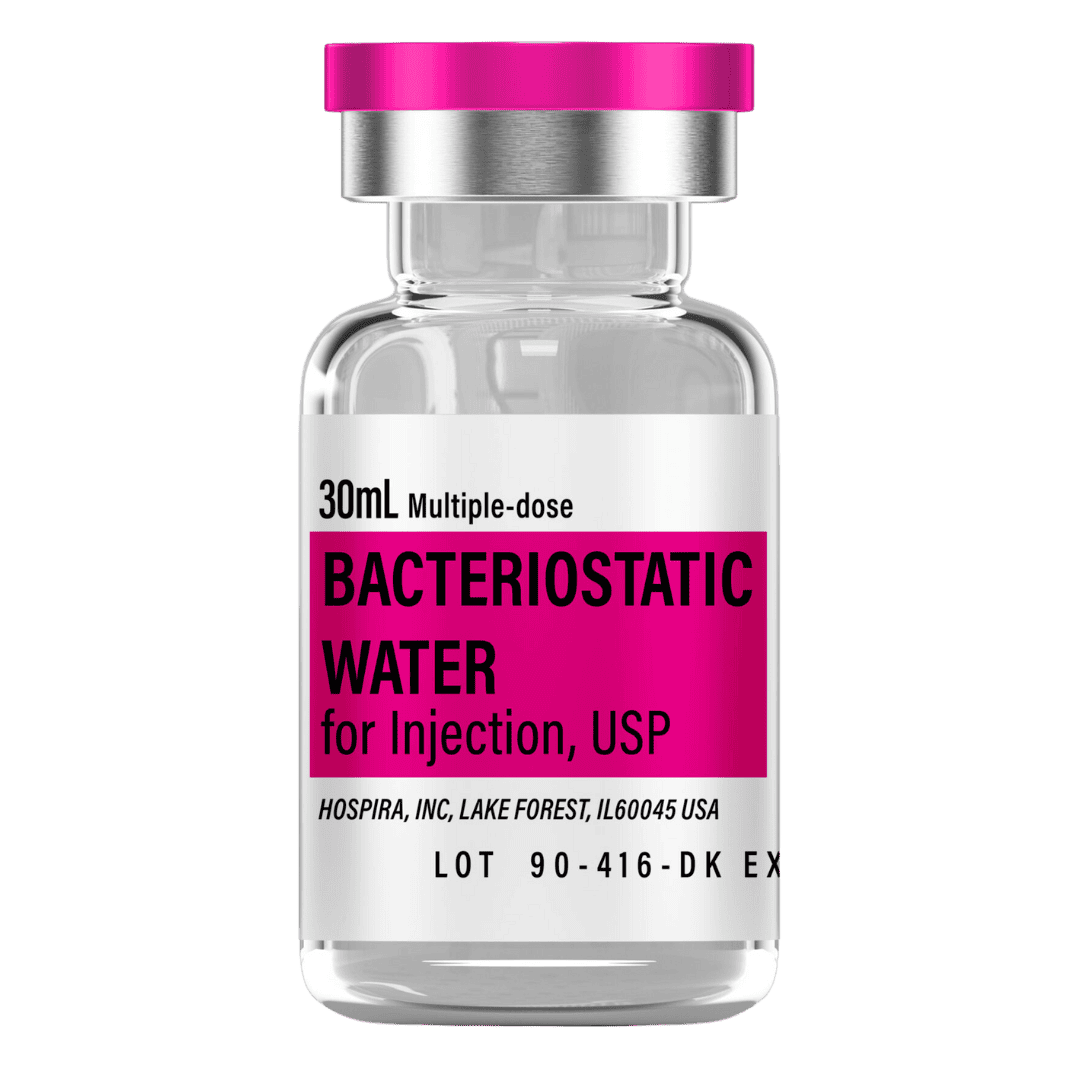
- Reconstitute: Add 2.0 mL bacteriostatic water → 5.0 mg/mL concentration.
- Typical weekly range: 2.5–15 mg once weekly (gradual 4‑week titration steps).
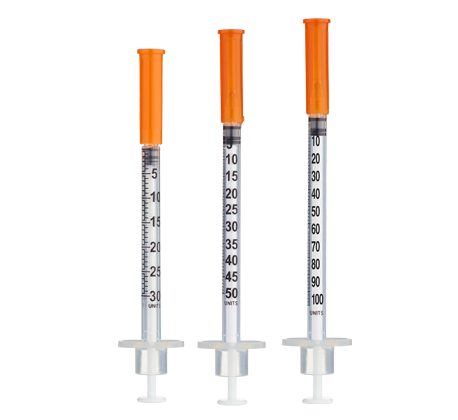
- Easy measuring: At 5.0 mg/mL, 1 unit = 0.01 mL = 50 mcg on a U‑100 insulin syringe.
- Storage: Lyophilized: freeze at −20 °C (−4 °F); after reconstitution, refrigerate at 2–8 °C (35.6–46.4 °F); use within 28 days.

Dosing & Reconstitution Guide
Subcutaneous, once weekly
Standard / Gradual Approach (2 mL = 5.0 mg/mL)
| Phase | Weekly Dose (mg) | Units (per injection) (mL) |
|---|---|---|
| Weeks 1–4 | 2.5 mg | 50 units (0.50 mL) × 1 injection |
| Weeks 5–8 | 5 mg | 100 units (1.0 mL) × 1 injection |
| Weeks 9–12 | 7.5 mg | 75 units (0.75 mL) × 2 injections |
| Weeks 13–16 | 10 mg | 100 units (1.0 mL) × 2 injections |
Frequency: Inject once weekly subcutaneously on the same day each week[1][5]. For doses requiring multiple injections, administer consecutively at different sites. Dose increases occur every 4 weeks to minimize gastrointestinal side effects[1]. Higher doses (12.5–15 mg/week) may be used in subsequent phases if tolerated and clinically indicated.
Reconstitution Steps
- Draw 2.0 mL bacteriostatic water with a sterile syringe.
- Inject slowly down the vial wall; avoid foaming.
- Gently swirl/roll until dissolved (do not shake).
- Label with reconstitution date and refrigerate at 2–8 °C (35.6–46.4 °F), protected from light.
- Use within 28 days of reconstitution[6].
Supplies Needed
Plan based on an 8–16 week protocol with gradual titration (once‑weekly dosing).
-
Peptide Vials (Tirzepatide, 10 mg each):
- 8 weeks (2.5→5 mg/wk): ~30 mg total ≈ 3 vials
- 12 weeks (2.5→7.5 mg/wk): ~60 mg total ≈ 6 vials
- 16 weeks (2.5→10 mg/wk): ~100 mg total ≈ 10 vials
-
Insulin Syringes (U‑100, 1 mL):
- 8 weeks: 8 syringes (1/week)
- 12 weeks: 16 syringes (~1.3/week avg)
- 16 weeks: 24 syringes (~1.5/week avg)
-
Bacteriostatic Water (10 mL bottles): Use 2.0 mL per vial for reconstitution.
- 8 weeks (3 vials): 6 mL → 1 × 10 mL bottle
- 12 weeks (6 vials): 12 mL → 2 × 10 mL bottles
- 16 weeks (10 vials): 20 mL → 2 × 10 mL bottles
-
Alcohol Swabs: One for the vial stopper + one for the injection site each administration day.
- Per week: 2 swabs (1 injection day)
- 8 weeks: 16 swabs → recommend 1 × 100‑count box
- 12 weeks: 24 swabs → recommend 1 × 100‑count box
- 16 weeks: 32 swabs → recommend 1 × 100‑count box
Protocol Overview
Concise summary of the once‑weekly regimen.
- Goal: Support glycemic control, weight management, and metabolic health through dual incretin receptor activation[2].
- Schedule: Weekly subcutaneous injection on the same day each week for 12–16+ weeks.
- Dose Range: 2.5–15 mg weekly with 4‑week titration intervals.
- Reconstitution: 2.0 mL per 10 mg vial (5.0 mg/mL) for manageable injection volumes.
- Storage: Lyophilized frozen; reconstituted refrigerated for up to 28 days.
Dosing Protocol
Suggested weekly titration approach.
- Start: 2.5 mg once weekly for 4 weeks (initiation dose)[1].
- Escalate: Increase by 2.5 mg every 4 weeks as tolerated.
- Maintenance: 5–15 mg weekly based on response and tolerability.
- Frequency: Once per week (subcutaneous), same day each week.
- Timing: Any time of day; with or without food; rotate injection sites.
Storage Instructions
Proper storage preserves peptide quality.
- Lyophilized: Store at −20 °C (−4 °F) in dry, dark conditions; minimize moisture exposure.
- Reconstituted: Refrigerate at 2–8 °C (35.6–46.4 °F); do not freeze reconstituted solution[6].
- Shelf life: Use reconstituted solution within 28 days[6].
- Allow vials to reach room temperature before opening to reduce condensation uptake.
Important Notes
Practical considerations for consistency and safety.
- Use new sterile insulin syringes; dispose in a sharps container[7].
- Rotate injection sites (abdomen, thighs, upper arms) to reduce local irritation[8].
- For multi‑injection doses, use different sites for each injection on the same day.
- Inject slowly; wait a few seconds before withdrawing the needle.
- Document weekly dose, date, and injection site to maintain consistency.
- Gastrointestinal effects (nausea, diarrhea) are common initially; gradual titration helps minimize them[1].
How This Works
Tirzepatide is a novel dual agonist that simultaneously activates GLP‑1 (glucagon‑like peptide‑1) and GIP (glucose‑dependent insulinotropic polypeptide) receptors[1][2]. This dual mechanism enhances glucose‑dependent insulin secretion while suppressing glucagon release, slowing gastric emptying, and promoting satiety through central appetite regulation[2]. The added GIP activity appears to synergistically amplify metabolic effects beyond GLP‑1 alone, contributing to superior weight reduction observed in clinical trials[3][4]. Its ~5‑day half‑life enables convenient once‑weekly administration[1].
Potential Benefits & Side Effects
Observations from clinical trials and published literature.
- Glycemic control: Significant HbA1c reductions in type 2 diabetes trials[4][9].
- Weight reduction: Clinical trials report substantial body‑weight loss (up to ~11 kg more than GLP‑1 RA comparators over 26 weeks at higher doses)[3][4].
- Cardiovascular markers: Improvements in lipid profiles and blood pressure observed in some studies[9].
- Common side effects: Gastrointestinal (nausea, diarrhea, vomiting, constipation) — typically mild‑to‑moderate and dose‑dependent; gradual titration reduces incidence[1][5].
- Injection‑site reactions: Occasional mild redness or irritation at subcutaneous injection sites.
Lifestyle Factors
Complementary strategies for best outcomes.
- Pair with a balanced, calorie‑appropriate diet; reduced appetite may naturally decrease intake.
- Prioritize protein to preserve lean mass during weight loss.
- Combine resistance training and aerobic activity to support metabolic health.
- Stay hydrated, especially given potential gastrointestinal effects.
- Prioritize sleep and stress management to support adherence and recovery.
Injection Technique
General subcutaneous guidance from clinical best‑practice resources[8][10].
- Clean the vial stopper and skin with alcohol; allow to dry.
- Pinch a skinfold; insert the needle at 45–90° into subcutaneous tissue[8][10].
- Do not aspirate for subcutaneous injections; inject slowly and steadily[10].
- Rotate sites systematically (abdomen avoiding 2‑inch radius around navel, outer thighs, upper arms) to avoid lipohypertrophy[8].
- Dispose of needles and syringes in a sharps container immediately after use[7].
Recommended Source
We recommend Pure Lab Peptides for high‑purity Tirzepatide (10 mg).
Why Pure Lab Peptides?
- High‑purity, third‑party‑tested lots with batch COAs.
- Consistent, ISO‑aligned handling and documentation.
- Reliable fulfillment to maintain cold‑chain integrity.
Product ID: Tirzepatide 10mg
Important Note
This content is intended for therapeutic educational purposes only and does not constitute medical advice, diagnosis, or treatment.
References
-
StatPearls (NCBI Bookshelf)
— Farzam K, Patel P. Tirzepatide. StatPearls Publishing; 2024. Comprehensive overview of pharmacology, dosing, and clinical use. -
Frontiers in Endocrinology
— Gallwitz B. GIP/GLP-1 receptor agonist tirzepatide for type 2 diabetes and obesity. Front Endocrinol. 2022;13:1004044. -
The Lancet
— Frias JP, et al. Efficacy and safety of LY3298176 (tirzepatide), a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes (Phase 2 trial). Lancet. 2018;392(10160):2180-2193. -
New England Journal of Medicine
— Jastreboff AM, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205-216. -
FDA Prescribing Information
— Mounjaro (tirzepatide) injection Prescribing Information. Eli Lilly and Company; 2022. -
GobyMeds Pharmacy
— Does Compounded Tirzepatide Need To Be Refrigerated? Storage guidelines for reconstituted peptides. -
CDC Injection Safety
— Preventing Unsafe Injection Practices. Guidelines for multi-dose vials and safe needle disposal. -
MedlinePlus Medical Encyclopedia
— Subcutaneous (SQ) injections: Technique, site rotation, and best practices. -
Mayo Clinic
— Tirzepatide (Subcutaneous route) – Drugs and Supplements. Clinical overview and patient information. -
CDC Vaccine Administration
— Subcutaneous injection technique: angle, site selection, and no aspiration guidance. -
Hospital Pharmacy (PubMed)
— Jordan MA, et al. Accurate measurement of small-volume parenterals with syringes. Hosp Pharm. 2021;56(3):165-171. -
NCBI Bookshelf
— Best practices for injection: asepsis, preparation, and administration techniques. -
Subcutaneous Drug Injection Review (PMC)
— Pharmacologic considerations of the subcutaneous route for drug delivery. -
Pure Lab Peptides
— Tirzepatide (10 mg) product page with quality documentation and batch COAs.