Thymosin Alpha-1 (5 mg Vial) Dosage Protocol
Quickstart Highlights
Thymosin Alpha-1 (Tα1) is a 28–amino acid peptide originally isolated from the thymus gland, recognized for its broad immunomodulatory properties[1]. It has been investigated as an immune enhancer in chronic viral infections (hepatitis B/C, HIV/AIDS) and critical illness (sepsis, COVID-19)[2][3]. This educational protocol presents a once‑daily subcutaneous approach using a practical dilution for clear insulin‑syringe measurements.
- Reconstitute: Add 3.0 mL bacteriostatic water → ~1.67 mg/mL concentration.
- Typical daily range: 300–500 mcg once daily (gradual titration).
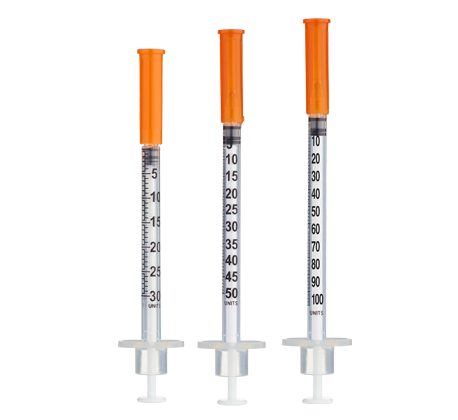
- Easy measuring: At 1.67 mg/mL, 1 unit = 0.01 mL ≈ 16.7 mcg on a U‑100 insulin syringe.
- Storage: Lyophilized: refrigerate at 2–8 °C (35.6–46.4 °F) or freeze at −20 °C (−4 °F); after reconstitution, refrigerate and use within 7 days.

Dosing & Reconstitution Guide
Educational guide for reconstitution and daily dosing
Standard / Gradual Approach (3 mL = ~1.67 mg/mL)
| Week | Daily Dose (mcg) | Units (per injection) (mL) |
|---|---|---|
| Week 1 | 300 mcg (0.3 mg) | 18 units (0.18 mL) |
| Weeks 2–8 | 500 mcg (0.5 mg) | 30 units (0.30 mL) |
Frequency: Inject once daily subcutaneously. This 8‑week protocol begins at 300 mcg to assess tolerance, then increases to a maintenance dose of 500 mcg daily from Week 2 onward. The 500 mcg daily dose yields ~3.5 mg/week, consistent with clinical dosing ranges[4][5]. Treatment durations of 8–16 weeks are commonly reported in literature.
Reconstitution Steps
- Draw 3.0 mL bacteriostatic water with a sterile syringe.
- Inject slowly down the vial wall; avoid foaming.
- Gently swirl/roll until dissolved (do not shake).
- Label with reconstitution date and refrigerate at 2–8 °C (35.6–46.4 °F), protected from light.
Supplies Needed
Plan based on an 8–16 week daily protocol with gradual titration.
-
Peptide Vials (Thymosin Alpha-1, 5 mg each):
- 8 weeks ≈ 6 vials
- 12 weeks ≈ 9 vials
- 16 weeks ≈ 12 vials
-
Insulin Syringes (U‑100):
- Per week: 7 syringes (1/day)
- 8 weeks: 56 syringes
- 12 weeks: 84 syringes
- 16 weeks: 112 syringes
-
Bacteriostatic Water (10 mL bottles): Use ~3.0 mL per vial for reconstitution.
- 8 weeks (6 vials): 18 mL → 2 × 10 mL bottles
- 12 weeks (9 vials): 27 mL → 3 × 10 mL bottles
- 16 weeks (12 vials): 36 mL → 4 × 10 mL bottles
-
Alcohol Swabs: One for the vial stopper + one for the injection site each day.
- Per week: 14 swabs (2/day)
- 8 weeks: 112 swabs → recommend 2 × 100‑count boxes
- 12 weeks: 168 swabs → recommend 2 × 100‑count boxes
- 16 weeks: 224 swabs → recommend 3 × 100‑count boxes
Protocol Overview
Concise summary of the once‑daily regimen.
- Goal: Support immune modulation and enhance host defense mechanisms[1][2].
- Schedule: Daily subcutaneous injections for 8–12 weeks (extend to 16 weeks if desired).
- Dose Range: 300–500 mcg daily with gradual titration.
- Reconstitution: 3.0 mL per 5 mg vial (~1.67 mg/mL) for accurate unit measurements.
- Storage: Lyophilized refrigerated or frozen; reconstituted refrigerated; use within 7 days.
Dosing Protocol
Suggested daily titration approach.
- Start: 300 mcg daily for Week 1 to assess tolerance.
- Target: 500 mcg daily from Week 2 onward.
- Frequency: Once per day (subcutaneous).
- Cycle Length: 8–12 weeks; optional extension to 16 weeks.
- Timing: Any consistent time; rotate injection sites.
Storage Instructions
Proper storage preserves peptide quality.
- Lyophilized: Store at 2–8 °C (35.6–46.4 °F) for short-term or −20 °C (−4 °F) for long-term; minimize moisture exposure[5].
- Reconstituted: Refrigerate at 2–8 °C (35.6–46.4 °F); use within 7 days when using bacteriostatic water; avoid freeze–thaw.
- Allow vials to reach room temperature before opening to reduce condensation uptake.
Important Notes
Practical considerations for consistency and safety.
- Use new sterile insulin syringes; dispose in a sharps container.
- Rotate injection sites (abdomen, thighs, upper arms) to reduce local irritation.
- Inject slowly; wait a few seconds before withdrawing the needle.
- Document daily dose and site rotation to maintain consistency.
- Inspect solution before each use—do not use if cloudy or discolored.
How This Works
Thymosin Alpha-1 is a naturally occurring thymic peptide that modulates immune function through multiple pathways[1]. It enhances the maturation and differentiation of T‑cells, augments dendritic cell function, and promotes the production of key cytokines including interferon‑α and interleukin‑2[2][6]. Clinical studies have demonstrated Tα1’s ability to enhance immune responses in immunocompromised individuals, including those with chronic hepatitis B and C infections[4]. Meta-analyses have also shown benefit in reducing mortality in moderate-to-critical COVID-19 patients[3].
Potential Benefits & Side Effects
Observations from preclinical and clinical literature.
- Supports enhanced T‑cell function and overall immune competence[1][2].
- Demonstrates an excellent safety profile; doses up to 1.6 mg twice weekly for 6–12 months have been well-tolerated[4][7].
- Even at experimental doses up to 16 mg SC over 12 months, no significant Tα1‑specific toxicity has been observed[7].
- Most common adverse effect is mild injection‑site irritation (redness or discomfort).
Lifestyle Factors
Complementary strategies for best outcomes.
- Maintain adequate sleep and stress management to support immune function.
- Consume a nutrient‑dense diet rich in vitamins C, D, and zinc.
- Engage in moderate physical activity to complement immune optimization.
- Avoid excessive alcohol and smoking, which impair immune responses.
Injection Technique
General subcutaneous guidance from clinical best‑practice resources[8].
- Clean the vial stopper and skin with alcohol; allow to dry.
- Pinch a skinfold; insert the needle at 45–90° into subcutaneous tissue[9][10].
- Do not aspirate for subcutaneous injections; inject slowly and steadily[9].
- Rotate sites systematically (abdomen, thighs, upper arms) to avoid lipohypertrophy[11].
Recommended Source
We recommend Pure Lab Peptides for high‑purity Thymosin Alpha-1 (5 mg).
Why Pure Lab Peptides?
- High‑purity, third‑party‑tested lots with batch COAs.
- Consistent, ISO‑aligned handling and documentation.
- Reliable fulfillment to maintain cold‑chain integrity.
Important Note
This content is for educational purposes only and is not medical advice.
References
-
World Journal of Virology
— Dominari et al. (2020): Comprehensive review of Thymosin Alpha-1 mechanisms, dosing (0.8–6.4 mg SC), and clinical applications -
Molecules (MDPI)
— Tao et al. (2023): Thymosin Alpha-1 in viral diseases—mechanisms and therapeutic applications in HBV, HCV, and HIV -
Inflammopharmacology
— Soeroto et al. (2023): Meta-analysis of Tα1 in COVID-19; significant mortality reduction in moderate-to-critical patients -
Expert Opinion on Biological Therapy
— Thymalfasin (Zadaxin) clinical overview: 1.6 mg SC twice weekly for hepatitis B treatment -
Annals of the New York Academy of Sciences
— Thymosin Alpha-1: biological activities, clinical applications, and pharmacokinetics review -
Clinical Immunology
— Mechanisms of thymosin alpha-1 immunomodulation: T-cell maturation and cytokine production -
FDA Peptide Advisory Committee (2024)
— Safety data: Tα1 doses up to 16 mg SC for 12 months showed no significant toxicity -
Hospital Pharmacy
— Jordan et al. (2021): Small-volume injection accuracy; recommends ≥20% syringe capacity for precision -
CDC
— Vaccine administration: subcutaneous route (angle/site; no aspiration required) -
Johns Hopkins Arthritis Center
— How to give a subcutaneous injection: site selection, rotation, and technique -
NCBI Bookshelf
— Best practices for injection: asepsis, preparation, site rotation, and administration -
Subcutaneous Drug Injection Review (PMC)
— Pharmacologic considerations of the subcutaneous route -
Pure Lab Peptides
— Thymosin Alpha-1 (5 mg) product page (quality and batch documentation)