Survodutide (10 mg Vial) Dosage Protocol
Quickstart Highlights
Survodutide (BI 456906) is an investigational dual GLP‑1/glucagon receptor agonist under development for obesity and metabolic liver disease[1][2]. In phase 2 trials, once‑weekly subcutaneous dosing (0.6–4.8 mg) produced dose‑dependent weight loss of 12.5–14.9% versus placebo over 46 weeks[1]. This educational protocol presents a once‑weekly subcutaneous titration approach using a practical dilution for clear insulin‑syringe measurements.
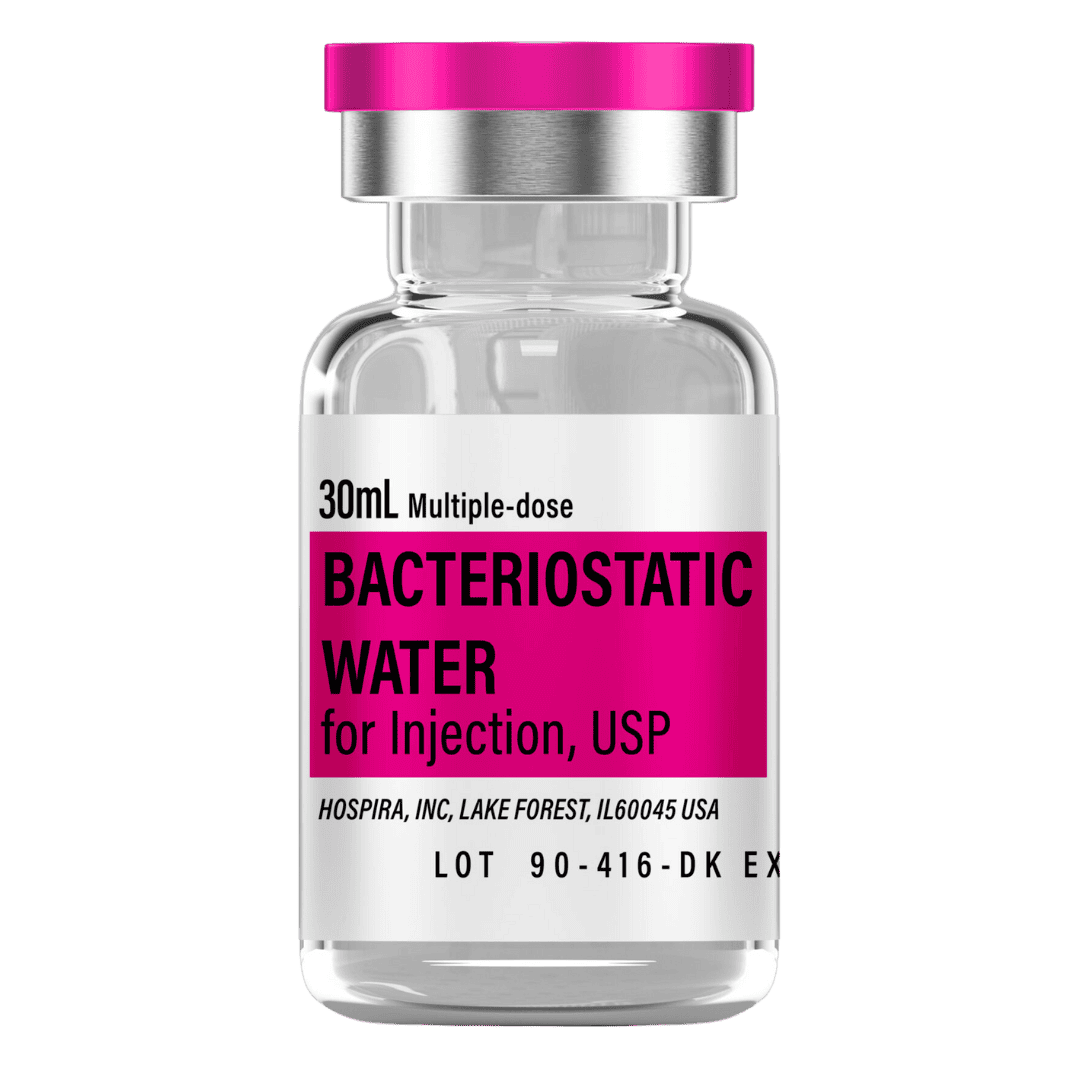
- Reconstitute: Add 2.0 mL bacteriostatic water → 5 mg/mL (5000 mcg/mL) concentration.
- Typical weekly range: 0.6–6.0 mg once weekly (gradual titration over 10–12 weeks).
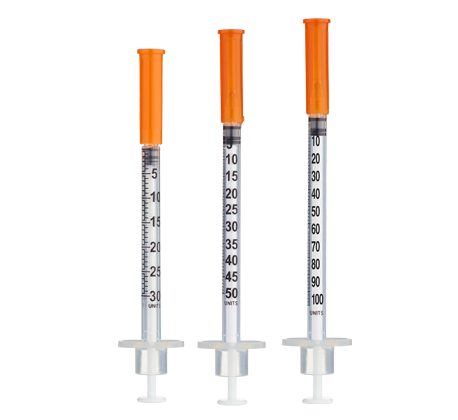
- Easy measuring: At 5 mg/mL, 1 unit = 0.01 mL = 50 mcg on a U‑100 insulin syringe.
- Storage: Lyophilized: freeze at ≤−20 °C (≤−4 °F); after reconstitution, refrigerate at 2–8 °C (35.6–46.4 °F); protect from light.

Dosing & Reconstitution Guide
Educational guide for reconstitution and weekly dosing
Standard / Gradual Titration (2 mL = 5 mg/mL)
Route & Frequency: Subcutaneous injection once weekly (all clinical trials used weekly dosing)[1][2].
| Week | Weekly Dose (mg) | Weekly Dose (mcg) | Units (mL) |
|---|---|---|---|
| Weeks 1–2 | 0.6 mg | 600 mcg | 12 units (0.12 mL) |
| Weeks 3–4 | 1.2 mg | 1200 mcg | 24 units (0.24 mL) |
| Weeks 5–6 | 1.8 mg | 1800 mcg | 36 units (0.36 mL) |
| Weeks 7–8 | 2.4 mg | 2400 mcg | 48 units (0.48 mL) |
| Weeks 9–10 | 3.6 mg | 3600 mcg | 72 units (0.72 mL) |
| Weeks 11–12 | 4.8 mg | 4800 mcg | 96 units (0.96 mL) |
| Week 13+ | 6.0 mg | 6000 mcg | 120 units (1.20 mL)* |
*Note: The 6.0 mg maintenance dose (120 units / 1.20 mL) exceeds a standard 100‑unit (1 mL) insulin syringe. Use a 1 mL or 3 mL Luer‑lock syringe with appropriate needle, or administer as two separate injections.
Reconstitution Steps
- Draw 2.0 mL bacteriostatic water with a sterile syringe.
- Inject slowly down the vial wall; avoid foaming.
- Gently swirl/roll until dissolved (do not shake).
- Label and refrigerate at 2–8 °C (35.6–46.4 °F), protected from light.
Supplies Needed
Plan based on an 8–16 week weekly protocol with gradual titration.
-
Peptide Vials (Survodutide, 10 mg each):
- 8 weeks ≈ 2 vials (~17 mg total)
- 12 weeks ≈ 4 vials (~39 mg total)
- 16 weeks ≈ 7 vials (~63 mg total)
-
Insulin Syringes (U‑100) or 1 mL Luer‑lock Syringes:
- Per week: 1 syringe (once weekly)
- 8 weeks: 8 syringes
- 12 weeks: 12 syringes
- 16 weeks: 16 syringes
-
Bacteriostatic Water (10 mL bottles): Use 2.0 mL per vial for reconstitution.
- 8 weeks (2 vials): 4 mL → 1 × 10 mL bottle
- 12 weeks (4 vials): 8 mL → 1 × 10 mL bottle
- 16 weeks (7 vials): 14 mL → 2 × 10 mL bottles
-
Alcohol Swabs: One for the vial stopper + one for the injection site each week.
- Per week: 2 swabs
- 8 weeks: 16 swabs
- 12 weeks: 24 swabs
- 16 weeks: 32 swabs → recommend 1 × 100‑count box
Protocol Overview
Concise summary of the once‑weekly regimen.
- Goal: Support metabolic improvement and weight management through dual GLP‑1/glucagon receptor activation[1][3].
- Schedule: Weekly subcutaneous injections for 12–16 weeks (or longer as studied in phase 3 trials).
- Dose Range: 0.6–6.0 mg weekly with gradual titration over 10–12 weeks.
- Reconstitution: 2.0 mL per 10 mg vial (5 mg/mL) for accurate unit measurements.
- Storage: Lyophilized frozen; reconstituted refrigerated; protect from light and avoid freeze–thaw.
Dosing Protocol
Suggested weekly titration approach based on clinical trial designs[1][2].
- Start: 0.6 mg weekly; increase by 0.6 mg every 2 weeks as tolerated.
- Target: 4.8–6.0 mg weekly by weeks 11–13.
- Frequency: Once per week (subcutaneous).
- Cycle Length: 12–16 weeks minimum; phase 3 trials extend to 48–72 weeks.
- Timing: Same day each week; rotate injection sites.
Storage Instructions
Proper storage preserves peptide quality.
- Lyophilized: Store at ≤−20 °C (≤−4 °F) in dry, dark conditions; minimize moisture exposure.
- Reconstituted: Refrigerate at 2–8 °C (35.6–46.4 °F); protect from light and avoid repeated freeze–thaw cycles.
- Allow vials to reach room temperature before opening to reduce condensation uptake.
Important Notes
Practical considerations for consistency and safety.
- Use new sterile syringes; dispose in a sharps container.
- Rotate injection sites (abdomen, thighs, upper arms) to reduce local irritation.
- Inject slowly; wait a few seconds before withdrawing the needle.
- Document weekly dose, injection site, and any tolerability observations.
- For doses exceeding 100 units (1.0 mL), consider using a 1 mL Luer‑lock syringe or splitting into two injections.
How This Works
Survodutide is a dual agonist that activates both GLP‑1 and glucagon receptors[1]. The GLP‑1 component enhances satiety and improves glycemic control, while the glucagon component increases energy expenditure and promotes hepatic lipid oxidation[3]. This dual mechanism is hypothesized to provide additive or synergistic effects on body weight and liver fat beyond GLP‑1 agonism alone[4]. In phase 2 trials, survodutide demonstrated substantial weight loss alongside improvements in liver enzyme markers[1][2].
Potential Benefits & Side Effects
Observations from clinical literature.
- Dose‑dependent weight loss of 12.5–14.9% (vs placebo) over 46 weeks in phase 2 obesity trials[1].
- Improvements in liver‑related biomarkers in NAFLD/NASH populations[2].
- Meta‑analysis confirms consistent weight‑loss efficacy across multiple randomized controlled trials[5].
- Adverse effects are primarily gastrointestinal: nausea, vomiting, diarrhea, and constipation (most common during dose escalation)[1][2].
- Gradual titration helps mitigate GI tolerability issues.
Lifestyle Factors
Complementary strategies for best outcomes.
- Pair with a balanced, protein‑forward diet tailored to energy needs; GLP‑1 agonists can reduce appetite significantly.
- Combine resistance training and aerobic activity to preserve lean mass during weight loss.
- Stay well hydrated, especially if experiencing GI side effects.
- Prioritize sleep and stress management to support metabolic health and adherence.
Injection Technique
General subcutaneous guidance from clinical best‑practice resources[9].
- Clean the vial stopper and skin with alcohol; allow to dry.
- Pinch a skinfold; insert the needle at 45–90° into subcutaneous tissue[7][8].
- Do not aspirate for subcutaneous injections; inject slowly and steadily[7].
- Rotate sites systematically (abdomen, thighs, upper arms) to avoid lipohypertrophy[10].
Recommended Source
We recommend Pure Lab Peptides for high‑purity Survodutide (10 mg).
Why Pure Lab Peptides?
- High‑purity, third‑party‑tested lots with batch COAs.
- Consistent, ISO‑aligned handling and documentation.
- Reliable fulfillment to maintain cold‑chain integrity.
Important Note
This content is intended for therapeutic educational purposes only and does not constitute medical advice, diagnosis, or treatment.
References
-
Lancet Diabetes & Endocrinology (2024)
— Le Roux et al. Phase 2 trial of once‑weekly SC survodutide (0.6–4.8 mg) in obesity; dose‑dependent weight loss of 12.5–14.9% -
Journal of Hepatology (2023)
— Guo et al. Efficacy, tolerability, and pharmacokinetics of survodutide in NAFLD/NASH patients (cirrhosis cohort) -
Obesity (Silver Spring) (2024)
— Wharton et al. SYNCHRONIZE‑1 & 2 phase 3 trial design for survodutide in obesity -
JACC: Heart Failure (2024)
— Kosiborod et al. SYNCHRONIZE‑CVOT design: survodutide in patients at cardiovascular risk -
Diabetology & Metabolic Syndrome (2024)
— Wan et al. Meta‑analysis of survodutide weight‑loss efficacy across randomized controlled trials -
Nature Reviews Endocrinology (2023)
— GLP‑1/glucagon dual agonism: mechanistic overview and therapeutic potential -
CDC
— Vaccine administration: subcutaneous route (angle/site guidance; no aspiration required) -
CDC (Subcut Injection PDF)
— Technique diagram and site guidance for subcutaneous injections -
MedlinePlus (NIH)
— Giving an insulin injection: patient guide for subcutaneous technique -
Veterans Affairs (2023)
— Insulin injection: how to use and where to inject (injection sites, rotation guidance) -
NCBI Bookshelf
— Best practices for injection (asepsis, preparation, and administration) -
Subcutaneous Drug Injection Review (PMC)
— Pharmacologic considerations of the subcutaneous route -
Pure Lab Peptides
— Survodutide (10 mg) product page (quality and batch documentation)