NAD+ (500 mg Vial) Dosage Protocol
Quickstart Highlights
NAD+ (nicotinamide adenine dinucleotide) is a critical coenzyme involved in cellular energy metabolism, DNA repair, and mitochondrial function[1]. Clinical research has primarily employed intravenous infusions at high doses, though subcutaneous administration at lower doses is emerging as a practical maintenance route[2][3]. This educational protocol presents a once‑daily subcutaneous approach with gradual titration for improved tolerability.
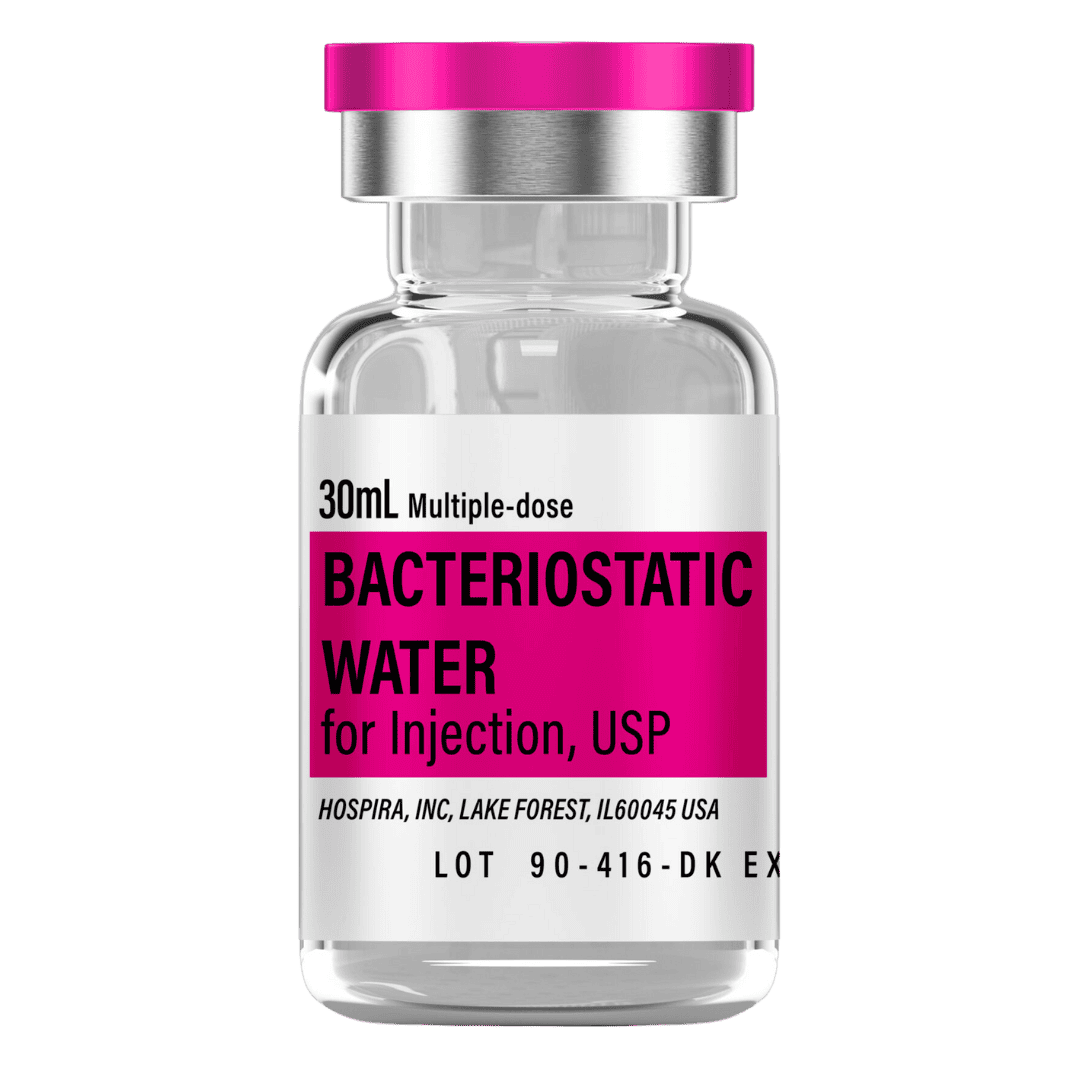
- Reconstitute: Add 3.0 mL bacteriostatic water → 166.7 mg/mL concentration.
- Typical daily range: 50–100 mg once daily subcutaneously (gradual titration from lower doses).
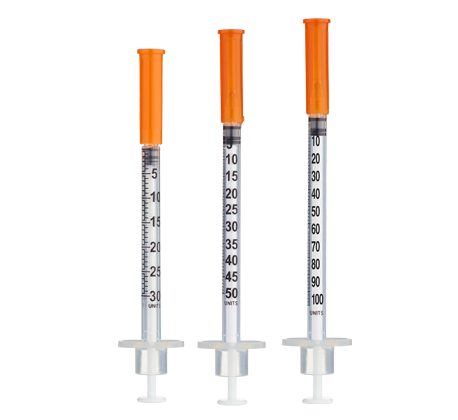
- Easy measuring: At 166.7 mg/mL, 1 unit = 0.01 mL = 1.67 mg on a U‑100 insulin syringe.
- Storage: Lyophilized: freeze at −20 °C (−4 °F); after reconstitution, refrigerate at 2–8 °C (35.6–46.4 °F) for up to 14 days; protect from light and avoid freeze–thaw cycles.

Dosing & Reconstitution Guide
Educational guide for reconstitution and daily dosing
Standard / Gradual Titration Approach (3 mL = 166.7 mg/mL)
| Week | Daily Dose (mg) | Units (per injection) (mL) |
|---|---|---|
| Week 1 | 50 mg | 30 units (0.30 mL) |
| Week 2 | 75 mg | 45 units (0.45 mL) |
| Weeks 3–8 | 100 mg | 60 units (0.60 mL) |
| Weeks 9–12 | 100 mg | 60 units (0.60 mL) |
| Weeks 13–16 | 100 mg | 60 units (0.60 mL) |
Frequency: Inject once daily subcutaneously. This gradual titration protocol begins at 50 mg daily to assess tolerance, as starting too high can produce adverse reactions such as insomnia, anxiety, or fatigue[4]. Most individuals find doses in the 50–100 mg range well‑tolerated after ramp‑up[5]. The 3.0 mL dilution keeps per‑injection volumes practical and allows for accurate unit measurements on standard insulin syringes.
Reconstitution Steps
- Allow the lyophilized vial to reach room temperature before opening to minimize moisture condensation.
- Draw 3.0 mL bacteriostatic water (0.9% benzyl alcohol) with a sterile syringe.
- Inject slowly down the vial wall to avoid foaming; do not aim directly at the powder.
- Gently swirl or roll the vial until the powder fully dissolves (do not shake vigorously).
- The resulting solution should be clear and colorless. If discoloration or precipitate appears, discard.
- Label with the reconstitution date and refrigerate at 2–8 °C (35.6–46.4 °F), protected from light.
- Use within 14 days; inspect before each use for clarity.
Note: Each 0.01 mL (1 unit) contains approximately 1.67 mg of NAD+. Example conversions: 50 mg = 30 units; 75 mg = 45 units; 100 mg = 60 units.
Supplies Needed
Plan based on an 8–16 week daily protocol with gradual titration as outlined above.
-
Peptide Vials (NAD+, 500 mg each):
- 8 weeks ≈ 11 vials (total 5,075 mg used)
- 12 weeks ≈ 16 vials (total 7,875 mg used)
- 16 weeks ≈ 22 vials (total 10,675 mg used)
-
Insulin Syringes (U‑100, 1 mL):
- Per week: 7 syringes (1/day)
- 8 weeks: 56 syringes
- 12 weeks: 84 syringes
- 16 weeks: 112 syringes
-
Bacteriostatic Water (10 mL bottles): Use 3.0 mL per vial for reconstitution.
- 8 weeks (11 vials): 33 mL → 4 × 10 mL bottles
- 12 weeks (16 vials): 48 mL → 5 × 10 mL bottles
- 16 weeks (22 vials): 66 mL → 7 × 10 mL bottles
-
Alcohol Swabs: One for the vial stopper + one for the injection site each day.
- Per week: 14 swabs (2/day)
- 8 weeks: 112 swabs → recommend 2 × 100‑count boxes
- 12 weeks: 168 swabs → recommend 2 × 100‑count boxes
- 16 weeks: 224 swabs → recommend 3 × 100‑count boxes
Protocol Overview
Concise summary of the once‑daily subcutaneous regimen.
- Goal: Support cellular energy metabolism, DNA repair pathways, and mitochondrial function through exogenous NAD+ supplementation[1].
- Schedule: Daily subcutaneous injections for 8–16 weeks with gradual dose titration.
- Dose Range: 50–100 mg daily; start low (50 mg) and increase by ~25 mg weekly as tolerated.
- Reconstitution: 3.0 mL per 500 mg vial (166.7 mg/mL) for accurate unit measurements.
- Storage: Lyophilized powder frozen at −20 °C (−4 °F); reconstituted solution refrigerated at 2–8 °C (35.6–46.4 °F) for up to 14 days; protect from light.
Dosing Protocol
Suggested daily titration approach for subcutaneous administration.
- Start: 50 mg daily for Week 1 to assess individual tolerance.
- Escalation: Increase by 25 mg in Week 2 (75 mg daily); advance to 100 mg daily by Week 3 if well‑tolerated[4][5].
- Maintenance: Continue at 100 mg daily for Weeks 3–16 (or adjust based on response and tolerability).
- Frequency: Once per day, subcutaneous injection.
- Timing: Any consistent time of day; some users prefer morning administration.
- Site Rotation: Rotate injection sites systematically (abdomen, thighs, upper arms) to prevent tissue buildup.
Storage Instructions
Proper storage is critical to preserve NAD+ stability and potency.
- Lyophilized (Unopened): Store at −20 °C (−4 °F) or below (ideally −80 °C for multi‑year storage) in a dry, dark environment[6]. Minimize moisture exposure as NAD+ powder is hygroscopic.
- Reconstituted Solution: Refrigerate at 2–8 °C (35.6–46.4 °F) and use within approximately 14 days[7]. Keep protected from light (UV exposure degrades NAD+).
- Avoid Freeze–Thaw Cycles: Do not repeatedly freeze and thaw reconstituted NAD+ solution, as this reduces potency.
- Inspection: Before each use, inspect the solution for clarity. A fresh NAD+ solution should be clear and colorless. Discard if discoloration, cloudiness, or precipitate develops.
Important Notes
Practical considerations for consistency, safety, and optimal results.
- Use new, sterile insulin syringes for each injection; dispose of used needles in a proper sharps container.
- Rotate injection sites systematically (abdomen at least 2 inches from navel, outer thighs, back of upper arms) to reduce soreness and prevent lipohypertrophy.
- Clean both the vial stopper and injection site with alcohol swabs; allow skin to air‑dry before injecting.
- Inject slowly over 5–10 seconds to minimize tissue irritation; wait a few seconds before withdrawing the needle.
- Document daily dose, injection site, and any observed effects to maintain consistency and track tolerance.
- If persistent redness or a lump develops at injection sites, apply over‑the‑counter hydrocortisone cream and rotate to a different location.
- Starting at the low end (50 mg) is recommended to assess individual tolerance before escalating dose[4].
How This Works
NAD+ is a coenzyme central to redox reactions, energy metabolism (glycolysis, TCA cycle, oxidative phosphorylation), and cellular maintenance pathways including DNA repair and mitochondrial biogenesis[1]. Cellular NAD+ levels decline with age and metabolic stress, which may contribute to reduced mitochondrial function and impaired cellular resilience[8].
Clinical research on NAD+ therapy has primarily used intravenous infusions at high doses (500–1,000 mg) for applications such as addiction treatment and acute metabolic support[2][9]. A pilot metabolic study demonstrated that a 750 mg NAD+ IV infusion over 6 hours was well‑tolerated in humans, with rapid metabolic clearance and no acute toxicity[10]. However, IV administration requires clinical supervision and specialized equipment.
Subcutaneous (SC) or intramuscular (IM) injections at lower doses (tens to low hundreds of milligrams) are emerging as practical alternatives for maintenance therapy and wellness applications[3][11]. Compounded NAD+ can be administered SC in small volumes, and SC self‑injection is convenient for ongoing use[12]. Conservative protocols start around 50–100 mg per injection a few times per week; the present protocol uses daily SC administration with gradual titration to optimize individual tolerance and response.
Potential Benefits & Side Effects
Observations from preclinical models and clinical case reports.
Potential Benefits (Reported in Literature)
- May support cellular energy production and mitochondrial function through replenishment of NAD+ pools[1][8].
- Human case reports and pilot studies suggest benefits at 100–300 mg/day ranges for cognitive support and metabolic health[13].
- High‑dose IV protocols (500–1,500 mg daily for ~10 days) have been used in open‑label studies for substance use disorder, with reports of reduced cravings and improved mood in follow‑ups[2][9].
- No severe adverse events have been reported in published NAD+/NADH trials; side effects are generally mild[14].
Potential Side Effects
- High doses can produce adverse reactions such as insomnia, anxiety, or fatigue if escalated too quickly; gradual titration is advised[4].
- Mild injection‑site reactions (redness, itching, soreness) may occur with subcutaneous administration; typically resolve with site rotation.
- Some individuals report transient headache or flushing; these effects are generally dose‑dependent.
- Doses exceeding ~200–300 mg/day should generally be reserved for supervised therapeutic use due to potential for metabolic byproduct accumulation[15].
Lifestyle Factors
Complementary strategies to maximize NAD+ therapy outcomes.
- Nutrition: Consume a balanced diet rich in NAD+ precursors (niacin/vitamin B3, tryptophan) and support nutrients (B‑vitamins, magnesium). Consider foods that support methylation pathways.
- Exercise: Regular physical activity (both resistance training and aerobic exercise) naturally upregulates cellular NAD+ biosynthesis and enhances mitochondrial adaptations.
- Sleep: Prioritize 7–9 hours of quality sleep per night to support cellular repair processes and optimize circadian NAD+ metabolism.
- Stress Management: Chronic stress depletes NAD+ pools through activation of poly(ADP‑ribose) polymerases (PARPs); incorporate stress‑reduction practices like meditation or yoga.
- Avoid Excessive Alcohol: Alcohol metabolism consumes NAD+; moderate or eliminate alcohol intake during NAD+ protocols.
Injection Technique
General subcutaneous injection guidance based on CDC best‑practice resources and clinical literature[16][17].
Preparation
- Wash hands thoroughly with soap and water.
- Clean the vial stopper with an alcohol swab and allow to air‑dry.
- Select injection site (abdomen at least 2 inches from navel, outer thigh, or back of upper arm) and clean with alcohol swab; allow skin to air‑dry completely before injecting.
Needle Selection
- Use a fine, short needle such as a 28–31 gauge insulin syringe.
- Needle length of 5/16 to 1/2 inch (8–12 mm) is sufficient to reach subcutaneous tissue in most adults[16].
- Standard U‑100 insulin syringes (1 mL capacity) are appropriate for the volumes used in this protocol (0.30–0.60 mL per injection).
Injection Procedure
- Pinch a fold of skin between your thumb and forefinger to isolate subcutaneous tissue.
- Insert the needle at approximately a 45° angle into the pinched subcutaneous layer[16][17]. (For ultrashort 4 mm needles, a 90° angle without pinching may be used, but for typical 8–12 mm needles, 45° with pinched skin is recommended.)
- Do not aspirate for subcutaneous injections[16].
- Depress the plunger slowly and steadily to inject the NAD+ solution over 5–10 seconds. Injecting too rapidly may increase irritation.
- Wait a few seconds after full injection before withdrawing the needle at the same angle it was inserted.
- Apply a clean cotton swab or bandage with light pressure to the site; do not rub vigorously.
- Dispose of the used needle/syringe immediately in a proper sharps container.
Site Rotation
- Rotate injection sites systematically to prevent soreness, lipohypertrophy, or scar tissue buildup[18].
- Alternate between left/right sides of the abdomen or different quadrants if injecting daily.
- The abdomen (at least 2 inches away from the navel) is a common site for subcutaneous NAD+ because it has a good layer of subcutaneous fat and tends to be less sensitive.
- Outer thigh and back of upper arm are alternative sites; choose areas with adequate subcutaneous tissue.
Volume Considerations
- NAD+ injections in this protocol range from 0.30 mL (50 mg dose) to 0.60 mL (100 mg dose).
- Subcutaneous tissue can comfortably absorb up to about 1.5 mL per injection; the volumes used here are well within this limit[19].
- If higher individual doses are ever needed (above ~150 mg), consider splitting into two injections at different sites to enhance absorption and comfort.
Recommended Source
We recommend Pure Lab Peptides for high‑purity NAD+ (500 mg vials).
Why Pure Lab Peptides?
- High‑purity NAD+ (≥98%) with third‑party testing and batch Certificates of Analysis (COAs).
- Consistent, ISO‑aligned handling, sterile lyophilization, and comprehensive documentation.
- Reliable fulfillment with proper cold‑chain packaging to maintain product integrity during shipping.
- Transparent quality assurance and responsive customer support for research applications.
Important Note
This content is intended for therapeutic educational purposes only and does not constitute medical advice, diagnosis, or treatment. NAD+ is provided for research use only and is not intended for human consumption. Always consult with a qualified healthcare professional before beginning any new supplementation or therapeutic protocol. Individual responses may vary, and proper medical supervision is recommended for NAD+ therapy.
References
-
Pharmaceuticals (Basel)
— Clinical evidence for targeting NAD+ therapeutically: metabolic pathways and therapeutic potential -
Current Psychiatry Research and Reviews
— NAD+ and enkephalinase infusions attenuate burden in substance use disorder (pilot of 50 cases) -
Fagron Academy (US)
— NAD+ dosing review: routes, protocols, and case study insights from clinical practice -
Jinfiniti (NAD+ Dosage Chart)
— NAD+ injection dosage guidance: chart, timing, and safety considerations for titration -
Olympia Pharmacy
— NAD+ injection (Nicotinamide Adenine Dinucleotide): compounding pharmacy information and dosing guidance -
Sigma-Aldrich Product Information
— β‑Nicotinamide adenine dinucleotide (NAD+) product specification and stability data -
Empower Pharmacy
— NAD+ injection (lyophilized): compounding specifications and reconstitution guidance -
PMC (Pharmacology & Potential Implications of NAD+)
— Comprehensive review of NAD+ metabolism, aging, and therapeutic applications -
AgelessRx
— NAD+ injection clinical overview and administration protocols -
Frontiers in Aging Neuroscience
— A pilot study investigating changes in human plasma and urine NAD+ metabolome during 6‑hour IV infusion (750 mg dose) -
Pure Bio Labs
— NAD+ vial (500 mg): high‑purity peptide product specifications -
Fagron Academy (NAD+ Review)
— Subcutaneous and intramuscular NAD+ administration: emerging practices for maintenance therapy -
Jinfiniti (Dosage Chart)
— Human case reports: benefits at 100–300 mg/day ranges for cognitive and metabolic support -
American Journal of Physiology (PubMed)
— Evaluation of safety and effectiveness of NAD+ in different clinical conditions: a systematic review -
Fagron Academy
— Dosing considerations: regimens exceeding 200–300 mg/day reserved for supervised therapeutic use -
CDC Pink Book (Chapter 6)
— Vaccine administration: needle selection and injection technique for subcutaneous route -
CDC Subcutaneous Injection Guide
— Technique diagram and site guidance for subcutaneous injections (45–90° angle, no aspiration) -
NCBI Bookshelf
— Best practices for injections: asepsis, site preparation, administration, and rotation to prevent lipohypertrophy -
PMC (Subcutaneous Drug Injection Review)
— Literature review of factors influencing pain at injection site and volume considerations for subcutaneous route -
Pure Lab Peptides
— NAD+ 500 mg product page: quality specifications and batch documentation