GHRP-2 (10mg Vial) Dosage Protocol
Quickstart Highlights
GHRP-2 10mg dosage protocol aims to support growth hormone release and potential body composition benefits through multiple daily subcutaneous injections.
- Typical daily injections of 100–300 mcg, 1–3 times per day
- Gradual or advanced protocols depending on experience and goals
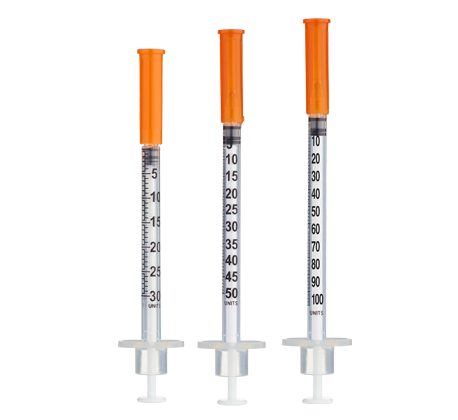
- Reconstitute to achieve practical measurement on insulin syringes
- Store lyophilized in a cool, dry place; refrigerate after mixing

Dosing & Reconstitution Guide
Educational guide for reconstitution and dosing protocol
Standard / Gradual Approach (3 mL = ~3,333 mcg/mL)
| Week | Daily Dosage (mcg) | Units (mL) |
|---|---|---|
| Weeks 1–2 | 100 mcg per injection, 2x daily (200 mcg total) | 3 units (0.03 mL) each injection |
| Weeks 3–4 | 150 mcg per injection, 2x daily (300 mcg total) | 4.5 units (0.045 mL) each injection |
| Weeks 5–8 | 200 mcg per injection, 2x daily (400 mcg total) | 6 units (0.06 mL) each injection |
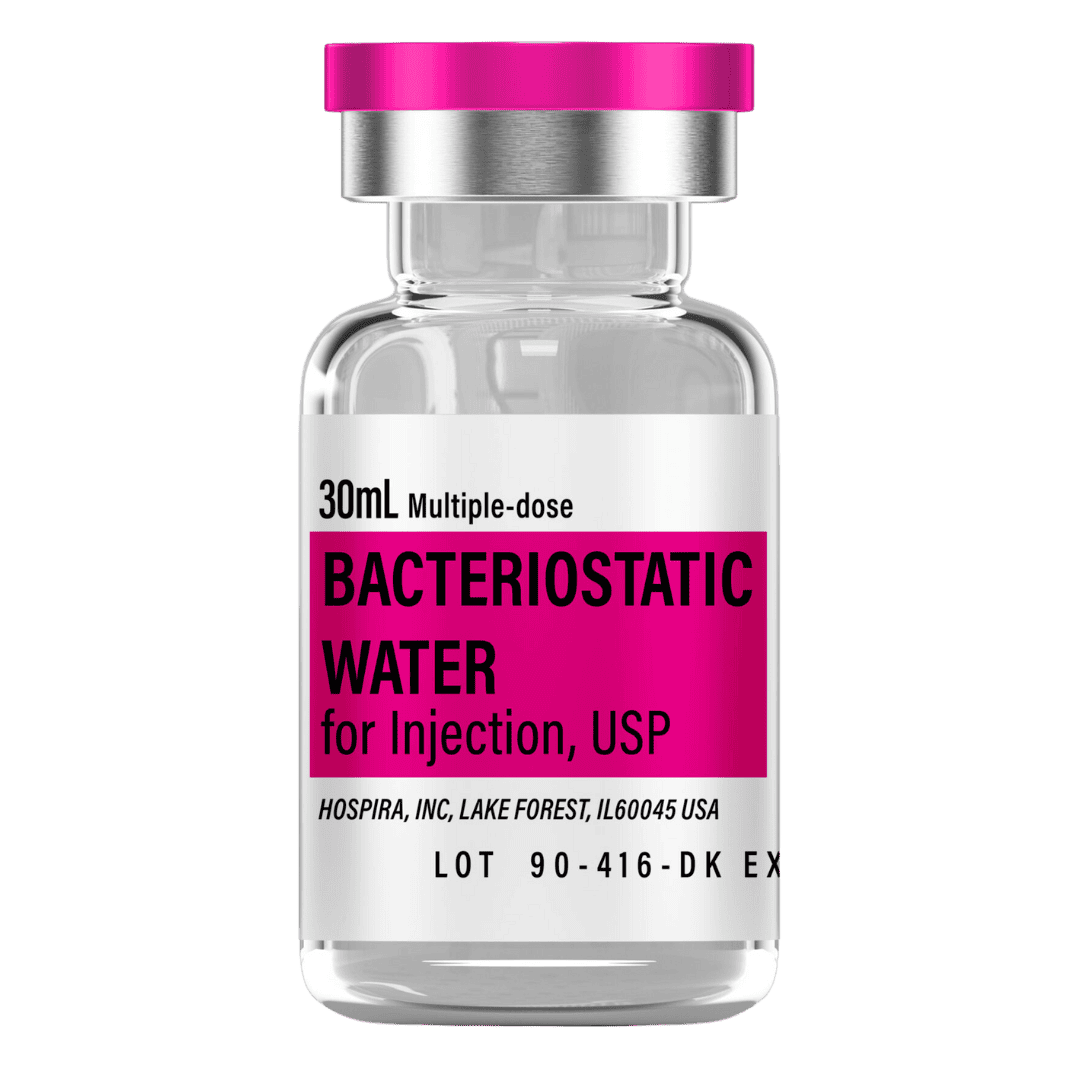
Reconstitute with 3 mL of bacteriostatic water for a solution of ~3,333 mcg/mL. Note that injection volumes remain under 10 units, so using a smaller insulin syringe (e.g., 50-unit or 30-unit) is recommended for better precision.
- Draw 3.0 mL of bacteriostatic water into a sterile syringe.
- Inject the water slowly along the vial wall to reduce foaming.
- Gently swirl—avoid vigorous shaking.
- Store the reconstituted solution at 2–8 °C (refrigerator), protected from light.
Advanced / Aggressive Approach (3 mL = ~3,333 mcg/mL)
| Week | Daily Dosage (mcg) | Units (mL) |
|---|---|---|
| Weeks 1–4 | 200 mcg per injection, 3x daily (600 mcg total) | 6 units (0.06 mL) each injection |
| Weeks 5–8 | 300 mcg per injection, 3x daily (900 mcg total) | 9 units (0.09 mL) each injection |
This approach suits experienced researchers targeting higher daily totals (≥600 mcg). Reconstitute with 3 mL of bacteriostatic water for ~3,333 mcg/mL. Using a smaller-volume insulin syringe is advised for accurate low-unit measurements.
- Draw 3.0 mL of bacteriostatic water into a sterile syringe.
- Slowly inject the water to minimize foam.
- Gently swirl the vial—avoid vigorous shaking.
- Store the reconstituted solution at 2–8 °C (refrigerator), protected from light.
Protocol Overview
A concise summary of this multi-daily injection regimen.
- Goal: Support natural growth hormone release
- Schedule: 1–3 subcutaneous injections per day
- Dose Range: ~100–300 mcg per injection
- Reconstitution: Up to 3 mL for convenient measuring (~3,333 mcg/mL)
- Storage: Keep lyophilized vials in a cool, dry place; refrigerate after mixing
Dosing Protocol
Suggested multi-daily injection strategy for consistent GH stimulation.
- Daily Dose: 200–900 mcg total (split into multiple injections)
- Frequency: 1–3 injections per day, spaced 6–8 hours apart
- Cycle Length: 8 weeks (extend to 12 weeks based on research goals)
- Meal Timing: Inject on an empty stomach; wait 30 minutes before eating
- Maximum Single Dose: ~300 mcg to minimize side effects
Storage Instructions
Proper storage ensures peptide integrity.
- Lyophilized: Store in a cool, dry place, away from direct light
- Reconstituted: Refrigerate at 2–8°C
- Use within 30 days of reconstitution
- Avoid repeated freeze-thaw cycles
Supplies Needed
Ensure you have these on hand for an 8–12 week research protocol.
- Peptide Vials (Product ID: 11587):
• 8 wks ≈ 1 vial
• 12 wks ≈ 2 vials
(More if using higher daily doses) - Insulin Syringes:
• 8 wks ≈ 2–3 per week
• 12 wks ≈ 2–3 per week
(Consider smaller 30-unit syringes if final injection volume is under 10 units) - Bacteriostatic Water: 1× 30ml
- Alcohol Swabs: 1 box
Important Notes
Practical tips to enhance safety and efficacy.
- Always use sterile insulin syringes & rotate injection sites.
- Injections on an empty stomach typically yield better results.
- Monitor for any adverse reactions; discontinue if concerns arise.
- Maintain a consistent schedule for maximum GH release.
How This Works
GHRP-2 (Growth Hormone Releasing Peptide-2) stimulates the release of endogenous growth hormone via ghrelin receptor activation.
- GH Secretion: Encourages the pituitary to secrete growth hormone
- Potential Synergy: Often combined with other GHRHs for amplified GH pulse
- Metabolic Impact: May influence appetite, fat metabolism, and recovery
Potential Benefits & Side Effects
Research suggests GHRP-2 may support GH-related pathways, though individual responses vary.
- May enhance lean body mass and support recovery
- Could support healthy GH and IGF-1 levels
- Potential side effects: increased appetite, mild water retention
- Rare side effects: nausea, lightheadedness, or injection-site irritation
Lifestyle Factors
Simple changes that can help optimize your protocol.
- Follow a protein-rich, balanced diet
- Incorporate resistance training and adequate rest
- Stay hydrated and manage stress levels
Injection Technique
Simple guidelines for safe daily injections.
- Clean vial rubber stopper & injection site with alcohol swabs
- Insert needle at a 45–90° angle into subcutaneous tissue
- Inject slowly & rotate sites (abdomen, thigh, etc.)
Recommended Source
We recommend Pure Lab Peptides for high-purity BPC-157 + TB-500 blend.
Why Pure Lab Peptides?
- Verifies ≥99% purity through independent lab testing
- Trusted by researchers seeking reliable results
- Follows rigorous manufacturing standards for consistent quality
Important Note
This guide is for educational purposes only. Always consult a qualified healthcare provider before starting or modifying any therapy.
References
-
University of Maryland Archive
– GHRP-2 final 2021 whitepaper -
PubMed
– GH secretion pattern study -
PMC
– Growth hormone secretagogues article -
PMC
– GHRP-2 appetite stimulation research -
Animal Bioscience
– GHRP-2 in livestock model study -
FDA Document
– Label info on GH-related drug -
PMC
– GH changes in older adults -
Journal of Endocrinology
– GHRP-2 mechanism analysis -
PubMed
– Growth hormone release study -
FDA
– Bulk drug substances warning -
WADA Document
– GH releasing factors overview -
J Clin Endocrinol Metab
– Endogenous GH release study -
Wiley JCSM
– GH clinical study findings -
WADA Document
– Growth Hormone Releasing Factors 2.0 -
Drugs.com
– Somatropin dosage guidelines -
PubMed
– GH secretion research in vivo -
PMC
– Clinical trials on GHRP-2 -
J Clin Endocrinol Metab
– GH secretion in human subjects -
PubMed
– GH axis functional analysis -
J Clin Endocrinol Metab
– GH pulse frequency study -
Journal of the Endocrine Society
– Recent findings on GH pathways -
PLOS ONE
– Clinical GHRP-2 evaluation -
Karger Journal
– Clinical & basic GH peptide study -
PMC
– GH & metabolic parameters review -
Am J Physiol Endocrinol Metab
– GH axis in endocrine research -
Frontiers in Endocrinology
– Comprehensive GH receptor review