Retatrutide (10 mg Vial) Dosage Protocol
Quickstart Highlights
Retatrutide is a novel triple agonist targeting GLP-1, GIP, and glucagon receptors[1], studied for substantial weight loss and metabolic improvement in obesity and type 2 diabetes[2][3]. With an extended half-life of approximately 6 days, this peptide enables convenient once-weekly subcutaneous dosing with a gradual titration protocol to optimize tolerability[1][4].
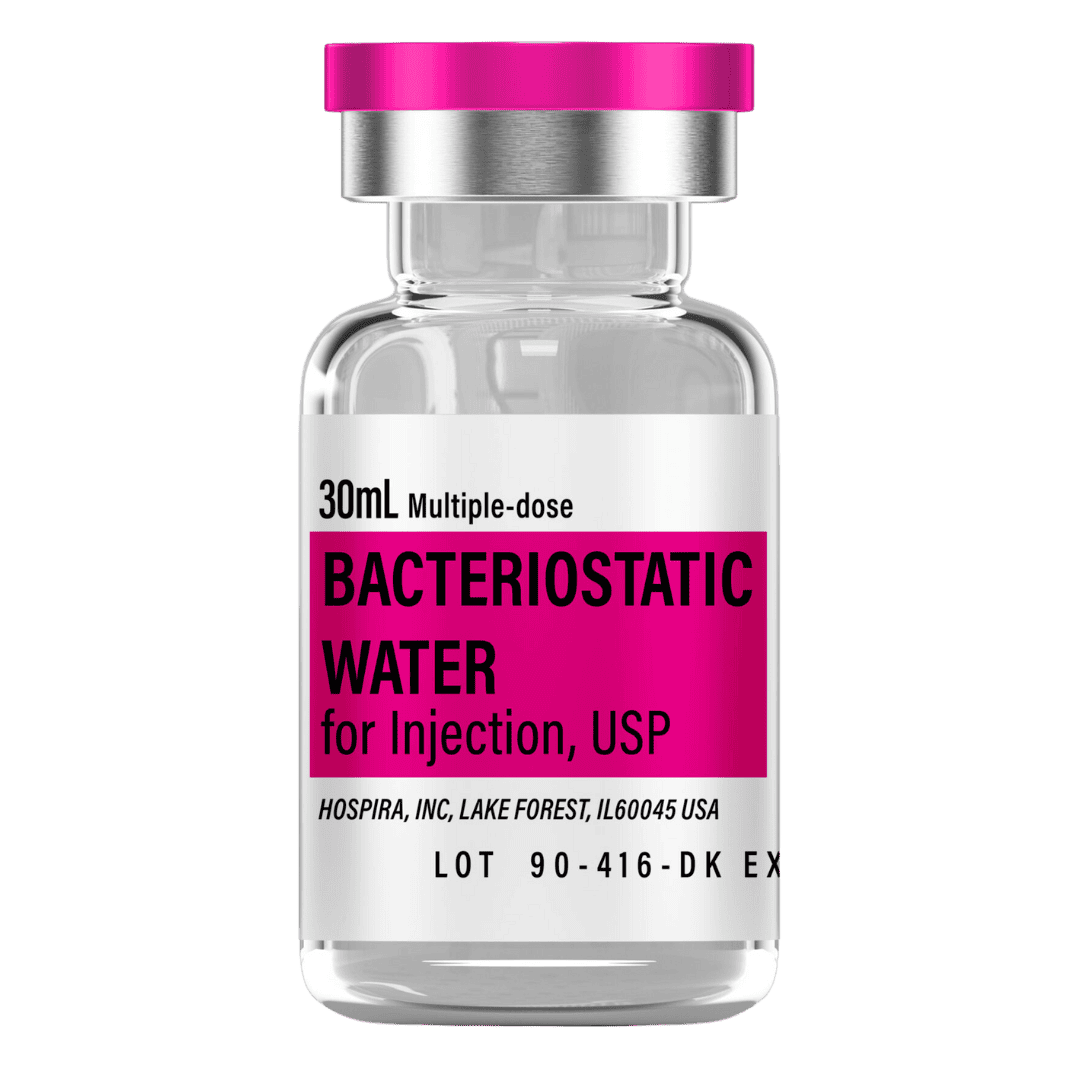
- Reconstitute: Add 2.0 mL bacteriostatic water → 5.0 mg/mL concentration for convenient weekly doses.
- Typical weekly range: 2–12 mg once weekly (gradual escalation over 12+ weeks).
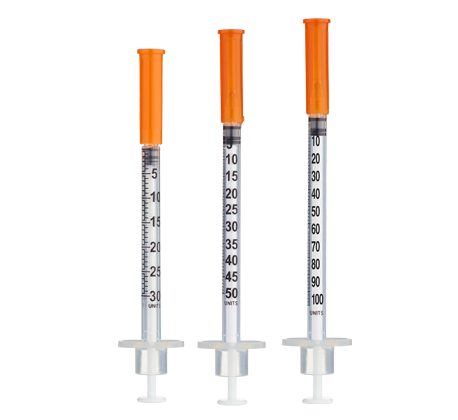
- Easy measuring: At 5.0 mg/mL, 1 unit = 0.01 mL ≈ 50 mcg on a U-100 insulin syringe.
- Storage: Lyophilized: freeze at −20 °C (−4 °F); after reconstitution, refrigerate at 2–8 °C (35.6–46.4 °F) and use within 2–4 weeks.

Dosing & Reconstitution Guide
Educational guide for reconstitution and weekly dosing
Standard / Gradual Titration Approach (2 mL = 5.0 mg/mL)
| Week(s) | Weekly Dose (mcg) (mg) | Units (per injection) (mL) |
|---|---|---|
| Weeks 1–4 | 2,000 mcg (2 mg) | 40 units (0.40 mL) |
| Weeks 5–8 | 4,000 mcg (4 mg) | 80 units (0.80 mL) |
| Weeks 9–12 | 8,000 mcg (8 mg) | 160 units (1.60 mL) — use 2 vials or split injection |
| Weeks 13+ | 12,000 mcg (12 mg) | 240 units (2.40 mL) — use 2 vials or split injection |
Frequency: Inject once weekly subcutaneously. This schedule follows clinical trial protocols that demonstrated significant weight loss (up to 24% at 48 weeks with 12 mg weekly)[2] while minimizing gastrointestinal adverse events through gradual escalation[1][4]. Starting at 2 mg weekly (versus 4 mg) significantly reduces initial GI side effects. For doses exceeding 1.0 mL, either reconstitute a second vial or split the dose into multiple injection sites.
Reconstitution Steps
- Draw 2.0 mL bacteriostatic water with a sterile syringe.
- Inject slowly down the vial wall; avoid foaming.
- Gently swirl/roll until dissolved (do not shake vigorously).
- Label with reconstitution date and refrigerate at 2–8 °C (35.6–46.4 °F), protected from light.
Note: The 10 mg vial size with 2.0 mL reconstitution provides excellent concentration (5.0 mg/mL) for convenient dosing. Lower weekly doses (2–4 mg) fit comfortably in a single syringe, while higher maintenance doses (8–12 mg) require multiple vials or split injections across different sites.
Supplies Needed
Plan based on a 12–48 week weekly protocol with gradual titration to maintenance dose.
-
Peptide Vials (Retatrutide, 10 mg each): Requirements increase with dose escalation
- 12 weeks (2→4→8 mg titration): ~6 vials
- 24 weeks (up to 12 mg): ~20 vials
- 48 weeks (maintenance at 12 mg): ~49 vials
-
Insulin Syringes (U-100, 1 mL):
- Per week: 1 syringe (once-weekly dosing)
- 12 weeks: 12 syringes
- 24 weeks: 24 syringes
- 48 weeks: 48 syringes
-
Bacteriostatic Water (10 mL bottles): Use 2.0 mL per vial for reconstitution.
- 12 weeks (6 vials): 12 mL → 2 × 10 mL bottles
- 24 weeks (20 vials): 40 mL → 4 × 10 mL bottles
- 48 weeks (49 vials): 98 mL → 10 × 10 mL bottles
-
Alcohol Swabs: One for the vial stopper + one for the injection site each week.
- Per week: 2 swabs
- 12 weeks: 24 swabs → 1 × 100-count box
- 24 weeks: 48 swabs → 1 × 100-count box
- 48 weeks: 96 swabs → 1 × 100-count box
Protocol Overview
Concise summary of the once-weekly regimen based on clinical trial protocols.
- Goal: Achieve substantial weight loss (up to 24% of body weight) and improved metabolic parameters through triple receptor agonism[2][5].
- Schedule: Weekly subcutaneous injections for 12+ weeks with gradual dose escalation.
- Dose Range: 2–12 mg once weekly; clinical trials tested up to 12 mg as the maximum maintenance dose[1][3].
- Reconstitution: 2.0 mL per 10 mg vial (5.0 mg/mL) provides excellent concentration for convenient weekly dosing.
- Storage: Lyophilized frozen at −20 °C (−4 °F); reconstituted solution refrigerated at 2–8 °C (35.6–46.4 °F); use within 2–4 weeks.
Dosing Protocol
Evidence-based weekly titration approach from clinical trials.
- Start: 2 mg once weekly for first 4 weeks to establish tolerability[1][4].
- Escalation: Increase to 4 mg weekly (Weeks 5–8), then 8 mg weekly (Weeks 9–12)[1].
- Maintenance: 12 mg weekly (Week 13 onward) for maximum efficacy; 8 mg weekly is an alternative maintenance dose[2].
- Frequency: Once per week (subcutaneous); consistent day/time recommended.
- Cycle Length: Minimum 12 weeks for titration; clinical trials extended to 48 weeks showing sustained weight loss[2].
- Timing: Any consistent weekly schedule; rotate injection sites each week.
Storage Instructions
Proper storage maintains peptide stability and potency.
- Lyophilized: Store at −20 °C (−4 °F) or colder for long-term preservation; protect from moisture and light[11][12].
- Reconstituted: Refrigerate at 2–8 °C (35.6–46.4 °F) immediately after mixing; use within 2–4 weeks for maximum potency[11].
- Handling: Allow frozen vials to reach room temperature before opening to minimize condensation; never expose reconstituted solution to heat or direct sunlight.
- Aliquoting: For extended storage beyond 4 weeks, consider freezing unused aliquots; avoid repeated freeze-thaw cycles[12].
Important Notes
Practical considerations for safe and effective weekly administration.
- Weekly consistency: Choose a specific day/time for your weekly injection and maintain this schedule throughout the protocol.
- Gradual titration is essential: Starting at 2 mg weekly (versus higher doses) significantly reduces initial gastrointestinal side effects[4].
- Use new sterile insulin syringes for each injection; dispose immediately in a puncture-proof sharps container[14].
- Rotate injection sites weekly (abdomen, thighs, upper arms) at least 1 inch apart to prevent local irritation or lipohypertrophy[15].
- For doses exceeding 1.0 mL, either reconstitute multiple vials or split the dose into separate injections at different sites.
- Monitor for gastrointestinal side effects (nausea, diarrhea); if severe, consider extending the time at current dose before escalating.
How This Works
Retatrutide is a first-in-class triple agonist that simultaneously activates three key metabolic hormone receptors: GLP-1 (glucagon-like peptide-1), GIP (glucose-dependent insulinotropic polypeptide), and glucagon[1][5]. This triple mechanism produces synergistic effects on appetite suppression, energy expenditure, and glucose metabolism that exceed single or dual agonists.
The GLP-1 component reduces appetite and slows gastric emptying; GIP enhances insulin secretion and may support fat metabolism; glucagon receptor activation increases energy expenditure and promotes fat oxidation[5][8]. With an extended half-life of approximately 6 days, retatrutide enables convenient once-weekly dosing while maintaining therapeutic levels[1].
In clinical trials, participants receiving 12 mg weekly retatrutide lost an average of 24% of their body weight over 48 weeks[2]. In adults with type 2 diabetes, retatrutide (up to 12 mg weekly) achieved approximately 17% weight loss at 36 weeks alongside HbA1c reductions of approximately 2.0% compared to placebo[3]. A 2025 meta-analysis of three trials (878 participants) confirmed retatrutide achieved significantly greater weight reduction than placebo (mean difference approximately 14% of body weight) with no significant increase in overall adverse events[6][7].
Potential Benefits & Side Effects
Observations from phase 2 and phase 3 clinical trials in humans.
Potential Benefits
- Substantial weight loss: Up to 24% reduction in body weight at 48 weeks with 12 mg weekly dosing[2].
- Glycemic improvement: Significant HbA1c reductions (approximately 2.0%) in adults with type 2 diabetes[3].
- Metabolic benefits: Improvements in lipid profiles, blood pressure, and cardiovascular risk markers[1][5].
- Convenient dosing: Once-weekly subcutaneous administration improves adherence compared to daily regimens[1].
Common Side Effects
- Gastrointestinal effects: Nausea, diarrhea, vomiting, and constipation are the most common adverse events; typically mild-to-moderate and diminish over time[2][4].
- Starting at 2 mg weekly (versus 4 mg) significantly reduces initial GI adverse events[4].
- Injection site reactions: Mild redness, swelling, or discomfort at injection sites may occur; rotate sites to minimize.
- Overall safety profile: Meta-analysis found no significant increase in overall adverse events compared to placebo[6][7].
Lifestyle Factors
Complementary strategies to optimize outcomes during retatrutide protocols.
- Nutrition: Adopt a balanced, protein-forward diet (1.0–1.2 g/kg body weight) to preserve lean mass during weight loss[10].
- Hydration: Maintain adequate fluid intake, especially during dose titration when GI effects are most common.
- Physical activity: Combine resistance training (2–3×/week) with moderate aerobic exercise to support metabolic adaptations and preserve muscle mass.
- Sleep & stress: Prioritize 7–9 hours of quality sleep and implement stress management techniques to support hormonal balance and adherence.
- Meal timing: Some individuals find smaller, more frequent meals help manage GI side effects during initial titration.
Injection Technique
Subcutaneous injection guidelines based on clinical best practices and CDC recommendations[13][14].
Pre-Injection Preparation
- Wash hands thoroughly with soap and water[14].
- Clean the vial stopper with an alcohol swab and allow to air dry.
- Select injection site (abdomen, thigh, or upper arm) and clean with a fresh alcohol swab; allow to dry completely[13].
- Draw prescribed dose carefully; check for air bubbles and expel if present.
Injection Procedure
- Pinch a skinfold of approximately 1 inch between thumb and forefinger[15].
- Insert needle at a 90-degree angle (45-degree if subcutaneous fat layer is thin) into the pinched skin[13][14].
- Do not aspirate for subcutaneous injections (aspiration is not required and may increase discomfort)[13].
- Inject slowly and steadily; depress plunger completely.
- Withdraw needle straight out and apply gentle pressure with clean gauze if needed.
Post-Injection Care
- Dispose of used syringe immediately in a puncture-proof sharps container; never recap needles[14].
- Return reconstituted vial to refrigerator promptly.
- Rotate injection sites weekly (at least 1 inch apart) to prevent lipohypertrophy[15].
- Monitor for excessive redness, swelling, or signs of infection at injection site.
Recommended Source
We recommend Pure Lab Peptides for high-purity Retatrutide (10 mg vials).
Why Pure Lab Peptides?
- Third-party tested: Each batch includes Certificate of Analysis (COA) verifying purity and composition.
- Consistent quality: ISO-aligned manufacturing and handling standards ensure reliable product integrity.
- Cold-chain integrity: Temperature-controlled shipping and storage throughout fulfillment process.
- Research-grade purity: Suitable for educational and research applications requiring high-quality peptides.
Product ID: 1061
Important Note
This content is intended for therapeutic educational purposes only and does not constitute medical advice, diagnosis, or treatment. Retatrutide is an investigational compound; consult qualified healthcare professionals before considering any peptide protocol. This information is for research and educational purposes only.
References
-
New England Journal of Medicine (2023)
— Triple-Hormone-Receptor Agonist Retatrutide for Obesity (Phase 2 trial; 48-week results) -
JAMA Network (2023)
— Retatrutide Phase 2 Obesity Trial: detailed efficacy and safety data (24% weight loss at 12 mg weekly) -
The Lancet (2023)
— Retatrutide in type 2 diabetes: Phase 2 trial (weight loss and glycemic outcomes at 36 weeks) -
PubMed / NEJM (2023)
— Retatrutide dosing and tolerability: starting at 2 mg vs 4 mg reduces GI adverse events -
Metabolites (PMC, 2025)
— Retatrutide—A Game Changer in Obesity Pharmacotherapy (comprehensive review of mechanism and trials) -
Baylor University Medical Center Proceedings (PMC, 2025)
— Efficacy and safety of retatrutide for obesity: meta-analysis of RCTs (878 participants) -
PubMed (2025)
— Meta-analysis: Retatrutide achieves ~14% greater weight reduction vs placebo with no increase in adverse events -
Molecular Metabolism (2025)
— Preclinical tri-agonist NN1706 (related compound): mechanism and pharmacokinetics -
Nature Reviews Endocrinology (2024)
— Triple agonist therapies for obesity and diabetes: clinical landscape review -
Journal of Cachexia, Sarcopenia and Muscle (PMC)
— Protein requirements during weight loss: preserving lean mass -
GenScript
— Peptide Storage and Handling Guidelines (technical bulletin for lyophilized and reconstituted peptides) -
Bachem
— Handling and Storage Guidelines for Peptides (technical article on stability and freeze-thaw cycles) -
Centers for Disease Control and Prevention (CDC)
— Vaccine Administration: Subcutaneous Injection (technique, angle, and site guidance) -
NCBI Bookshelf
— Injection Administration Best Practices (aseptic technique, sharps disposal, and safety) -
Pharmacologic Considerations of the Subcutaneous Route (PMC)
— Subcutaneous injection technique and site rotation to prevent lipohypertrophy -
Pure Lab Peptides
— Retatrutide (10 mg) product page (quality documentation and specifications)