BPC-157 + TB-500 (10 mg Blend) Dosage Protocol
Quickstart Highlights
This blend combines two well-studied regenerative peptides: BPC-157 (Body Protection Compound-157), a pentadecapeptide derived from human gastric juice with cytoprotective and wound-healing properties[1][2], and TB-500 (Thymosin Beta-4 fragment), a 43-amino-acid peptide involved in tissue repair, cell migration, and angiogenesis[3][4]. This educational protocol presents a once-daily subcutaneous approach using a practical dilution for clear insulin-syringe measurements.
- Reconstitute: Add 3.0 mL bacteriostatic water → 3.33 mg/mL total concentration (1.67 mg/mL of each peptide).
- Typical daily range: 600–1000 mcg total blend once daily (provides 300–500 mcg of each peptide).
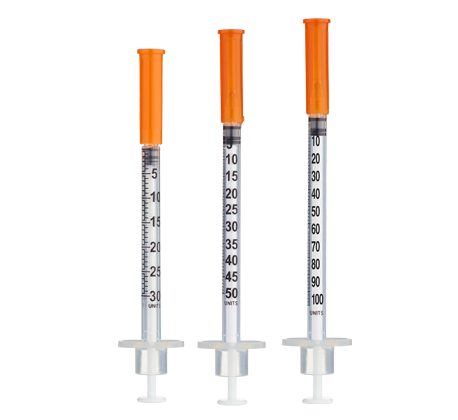
- Easy measuring: At 3.33 mg/mL, 1 unit = 0.01 mL ≈ 33.3 mcg total blend on a U-100 insulin syringe.
- Storage: Lyophilized: freeze at −20 °C (−4 °F); after reconstitution, refrigerate at 2–8 °C (35.6–46.4 °F); avoid freeze–thaw cycles.

Dosing & Reconstitution Guide
Educational guide for reconstitution and daily dosing
Standard / Gradual Approach (3 mL = ~3.33 mg/mL)
Route: Subcutaneous injection, once daily[5][6]. This blend contains equal amounts of BPC-157 and TB-500 (5 mg each); every dose drawn provides a 1:1 ratio of both peptides.
| Phase / Week | Daily Dose (Total Blend) | Each Peptide (mcg) | Units (mL) |
|---|---|---|---|
| Weeks 1–2 (Initial) | 600 mcg | 300 mcg BPC + 300 mcg TB-500 | 18 units (0.18 mL) |
| Weeks 3–4 (Loading) | 800 mcg | 400 mcg BPC + 400 mcg TB-500 | 24 units (0.24 mL) |
| Weeks 5–8 (Maintenance) | 600 mcg | 300 mcg BPC + 300 mcg TB-500 | 18 units (0.18 mL) |
Frequency: Inject once daily subcutaneously. This schedule uses 3.0 mL dilution to keep per-injection volumes ≥18 units for accurate measurement. Rotate injection sites systematically[7].
Reconstitution Steps
- Draw 3.0 mL bacteriostatic water with a sterile syringe.
- Inject slowly down the vial wall; avoid foaming or vigorous shaking.
- Gently swirl/roll until fully dissolved (do not shake).
- Label with date and refrigerate at 2–8 °C (35.6–46.4 °F), protected from light.
Advanced / Aggressive Approach (Acute Injury Support)
For acute tissue injuries, some protocols suggest a higher initial loading phase[8][9]. This approach uses higher daily doses during the first 4 weeks before tapering to maintenance. Use with caution; robust human dose-finding data remain limited.
| Phase / Week | Daily Dose (Total Blend) | Each Peptide (mcg) | Units (mL) |
|---|---|---|---|
| Weeks 1–2 (Aggressive Load) | 1000 mcg | 500 mcg BPC + 500 mcg TB-500 | 30 units (0.30 mL) |
| Weeks 3–4 (High Load) | 800 mcg | 400 mcg BPC + 400 mcg TB-500 | 24 units (0.24 mL) |
| Weeks 5–8 (Maintenance) | 600 mcg | 300 mcg BPC + 300 mcg TB-500 | 18 units (0.18 mL) |
Note: A small human case series combining BPC-157 and TB-500 for joint injuries reported improved outcomes at higher combined doses (4 mg BPC + 6 mg TB-500 intra-articular) compared to lower doses[8]. However, systemic subcutaneous protocols typically use the ranges above. Treatment duration is generally limited to 8–12 weeks before cycling off to evaluate response[9].
Supplies Needed
Plan based on an 8–16 week daily protocol with phased dosing (averaging ~700 mcg/day over the cycle).
-
Peptide Vials (BPC-157 + TB-500, 10 mg blend each):
- 8 weeks ≈ 4 vials
- 12 weeks ≈ 6 vials
- 16 weeks ≈ 8 vials
-
Insulin Syringes (U-100):
- Per week: 7 syringes (1/day)
- 8 weeks: 56 syringes
- 12 weeks: 84 syringes
- 16 weeks: 112 syringes
-
Bacteriostatic Water (10 mL bottles): Use 3.0 mL per vial for reconstitution.
- 8 weeks (4 vials): 12 mL → 2 × 10 mL bottles
- 12 weeks (6 vials): 18 mL → 2 × 10 mL bottles
- 16 weeks (8 vials): 24 mL → 3 × 10 mL bottles
-
Alcohol Swabs: One for the vial stopper + one for the injection site each day.
- Per week: 14 swabs (2/day)
- 8 weeks: 112 swabs → recommend 2 × 100-count boxes
- 12 weeks: 168 swabs → recommend 2 × 100-count boxes
- 16 weeks: 224 swabs → recommend 3 × 100-count boxes
Protocol Overview
Concise summary of the once-daily regimen.
- Goal: Support tissue repair, wound healing, and recovery from musculoskeletal injuries[1][3].
- Schedule: Daily subcutaneous injections for 8–12 weeks (extend to 16 weeks if needed).
- Dose Range: 600–1000 mcg total blend daily (300–500 mcg of each peptide).
- Reconstitution: 3.0 mL per 10 mg vial (~3.33 mg/mL) for accurate unit measurements.
- Storage: Lyophilized frozen; reconstituted refrigerated; avoid repeated freeze–thaw.
Dosing Protocol
Suggested daily phased approach.
- Start: 600 mcg total daily (300 mcg each peptide) for Weeks 1–2.
- Loading: Increase to 800 mcg daily for Weeks 3–4.
- Maintenance: Return to 600 mcg daily for Weeks 5–8+.
- Frequency: Once per day (subcutaneous).
- Cycle Length: 8–12 weeks; optional extension to 16 weeks.
Storage Instructions
Proper storage preserves peptide quality and stability.
- Lyophilized: Store at −20 °C (−4 °F) in dry, dark conditions; minimize moisture exposure.
- Reconstituted: Refrigerate at 2–8 °C (35.6–46.4 °F); use within 4–6 weeks; avoid freeze–thaw.
- Allow vials to reach room temperature before opening to reduce condensation uptake.
Important Notes
Practical considerations for consistency and safety.
- Both peptides are not approved for routine human use (sold for research purposes) and are banned in competitive sports[10].
- Use new sterile insulin syringes for each injection; dispose in a sharps container.
- Rotate injection sites (abdomen, thighs, upper arms) to reduce local irritation[7].
- Inject slowly; wait a few seconds before withdrawing the needle.
- Document daily dose and site rotation to maintain consistency.
How This Works
BPC-157 is a stable pentadecapeptide that promotes angiogenesis, modulates nitric oxide pathways, and demonstrates broad cytoprotective effects across gastrointestinal, musculoskeletal, and neurological tissues in preclinical models[1][2]. It has shown activity at very low doses (nanogram to microgram per kilogram) without demonstrable toxicity in animal studies[11].
TB-500 (Thymosin Beta-4) is an actin-sequestering peptide that promotes cell migration, wound healing, and anti-inflammatory responses[3][4]. It is well-tolerated in animal and early clinical studies, even at multi-milligram doses[12].
Combining these peptides may provide complementary mechanisms for tissue repair: BPC-157 for its trophic and anti-inflammatory effects, and TB-500 for enhanced cell migration and angiogenesis[8].
Potential Benefits & Side Effects
Observations from preclinical and limited clinical literature.
- May support accelerated healing of tendons, ligaments, muscles, and soft tissue injuries[1][5].
- Preclinical evidence suggests gastroprotective and anti-inflammatory properties for BPC-157[2].
- TB-500 promotes wound healing and may reduce scarring through enhanced cell migration[3].
- Both peptides are generally well tolerated; occasional mild injection-site reactions (redness, itching) may occur with subcutaneous administration.
- No significant toxicity has been documented up to high doses in animal studies[11][12].
Lifestyle Factors
Complementary strategies for optimal recovery outcomes.
- Prioritize adequate protein intake (1.6–2.2 g/kg body weight) to support tissue repair.
- Follow appropriate rehabilitation protocols for any injuries being addressed.
- Ensure adequate sleep (7–9 hours) to optimize recovery and tissue regeneration.
- Manage inflammation through balanced nutrition and stress reduction techniques.
Injection Technique
General subcutaneous guidance from clinical best-practice resources[6][7].
- Clean the vial stopper and skin with alcohol; allow to dry completely.
- Pinch a skinfold; insert the needle at 45–90° into subcutaneous tissue[13].
- Do not aspirate for subcutaneous injections; inject slowly and steadily[13].
- Rotate sites systematically (abdomen at least 2 inches from navel, thighs, upper arms, flank) to avoid lipohypertrophy[7].
- Apply gentle pressure post-injection; do not rub the site.
Recommended Source
We recommend Pure Lab Peptides for high-purity BPC-157 + TB-500 (10 mg blend).
Why Pure Lab Peptides?
- High-purity, third-party-tested lots with batch COAs.
- Consistent, ISO-aligned handling and documentation.
- Reliable fulfillment to maintain cold-chain integrity.
Important Note
This content is for educational purposes only and is not medical advice.
References
-
PubMed
— Preclinical safety evaluation of body protective compound-157, a potential drug for treating various wounds -
Current Pharmaceutical Design (PubMed)
— Stable gastric pentadecapeptide BPC-157: novel therapy in gastrointestinal tract -
PMC
— Utilizing developmentally essential secreted peptides such as Thymosin Beta-4 for regenerative therapies -
Annals of the New York Academy of Sciences (PubMed)
— Thymosin beta 4 and wound healing: new ideas -
PMC
— Emerging use of BPC-157 in orthopaedic sports medicine: a systematic review -
Johns Hopkins Arthritis Center
— How to give a subcutaneous injection -
PMC
— Subcutaneous drug delivery review: pharmacologic considerations -
ASIPP / Alternative Therapies in Health and Medicine
— Intra-articular injection of BPC-157 for multiple types of knee pain -
A4M
— Thymosin Beta-4 professional monograph -
PubMed
— Detection of thymosin beta-4 and related peptides in sport drug testing -
Journal of Physiology and Pharmacology (PubMed)
— BPC-157 activity at very low doses without toxicity in animal studies -
Expert Opinion on Biological Therapy (PubMed)
— Thymosin beta-4 and its role in wound healing -
CDC
— Vaccine administration: subcutaneous route (angle/site; no aspiration) -
NCBI Bookshelf
— Best practices for injection (asepsis, preparation, and administration) -
Pure Lab Peptides
— BPC-157 + TB-500 (10 mg blend) product page (quality and batch documentation)