TB-500 (10 mg Vial) Dosage Protocol
Quickstart Highlights
TB‑500 is a synthetic peptide fragment corresponding to the active region of thymosin beta‑4 (Tβ4), a naturally occurring 43‑amino‑acid protein involved in tissue repair and regeneration[1][2]. This educational protocol presents a once‑daily subcutaneous approach using a practical dilution for accurate insulin‑syringe measurements in research settings.
- Reconstitute: Add 3.0 mL bacteriostatic water → ~3.33 mg/mL concentration.
- Typical daily range: 500–1000 mcg once daily (gradual titration recommended).
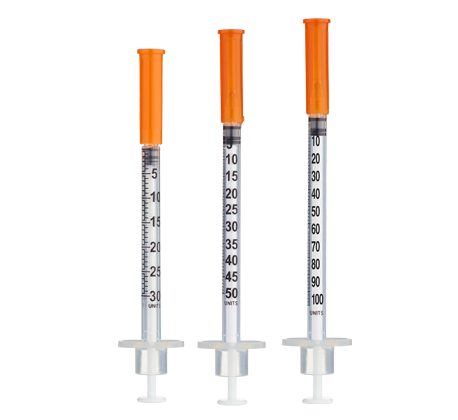
- Easy measuring: At 3.33 mg/mL, 1 unit = 0.01 mL ≈ 33.3 mcg on a U‑100 insulin syringe.
- Storage: Lyophilized: store at −20 °C (−4 °F); after reconstitution, refrigerate at 2–8 °C (35.6–46.4 °F); do not freeze reconstituted solution.

Dosing & Reconstitution Guide
Educational guide for reconstitution and daily dosing
Standard / Gradual Approach (3 mL = ~3.33 mg/mL)
| Phase | Daily Dose (mcg) | Units (per injection) (mL) |
|---|---|---|
| Weeks 1–2 | 500 mcg | 15 units (0.15 mL) |
| Weeks 3–4 | 600 mcg | 18 units (0.18 mL) |
| Weeks 5–8 | 750 mcg | 23 units (0.23 mL) |
| Weeks 9–12 | 1000 mcg | 30 units (0.30 mL) |
Frequency: Inject once daily subcutaneously. This schedule uses the largest practical dilution (3.0 mL) to keep per‑injection units in a comfortable range for accurate measurement. Total weekly dose averages ~5 mg, consistent with research protocols[3][4].
Reconstitution Steps
- Draw 3.0 mL bacteriostatic water with a sterile syringe.
- Inject slowly down the vial wall; avoid foaming.
- Gently swirl/roll until dissolved (do not shake).
- Label with date and concentration; refrigerate at 2–8 °C (35.6–46.4 °F), protected from light.
Supplies Needed
Plan based on an 8–16 week daily protocol with gradual titration.
-
Peptide Vials (TB‑500, 10 mg each):
- 8 weeks ≈ 4 vials
- 12 weeks ≈ 7 vials
- 16 weeks ≈ 10 vials
-
Insulin Syringes (U‑100):
- Per week: 7 syringes (1/day)
- 8 weeks: 56 syringes
- 12 weeks: 84 syringes
- 16 weeks: 112 syringes
-
Bacteriostatic Water (10 mL bottles): Use ~3.0 mL per vial for reconstitution.
- 8 weeks (4 vials): 12 mL → 2 × 10 mL bottles
- 12 weeks (7 vials): 21 mL → 3 × 10 mL bottles
- 16 weeks (10 vials): 30 mL → 3 × 10 mL bottles
-
Alcohol Swabs: One for the vial stopper + one for the injection site each day.
- Per week: 14 swabs (2/day)
- 8 weeks: 112 swabs → recommend 2 × 100‑count boxes
- 12 weeks: 168 swabs → recommend 2 × 100‑count boxes
- 16 weeks: 224 swabs → recommend 3 × 100‑count boxes
Protocol Overview
Concise summary of the once‑daily regimen.
- Goal: Support tissue repair, wound healing, and angiogenesis through the active thymosin beta‑4 fragment mechanism[5][6].
- Schedule: Daily subcutaneous injections for 8–12 weeks (extend to 16 weeks if research goals require).
- Dose Range: 500–1000 mcg daily with gradual titration (~5 mg/week average).
- Reconstitution: 3.0 mL per 10 mg vial (~3.33 mg/mL) for accurate unit measurements.
- Storage: Lyophilized frozen; reconstituted refrigerated; avoid freeze–thaw cycles of reconstituted solution.
Dosing Protocol
Suggested daily titration approach.
- Start: 500 mcg daily; increase by ~100–150 mcg every 2 weeks as tolerated.
- Target: 750–1000 mcg daily by Weeks 5–12.
- Frequency: Once per day (subcutaneous).
- Cycle Length: 8–12 weeks; optional extension to 16 weeks based on research protocol.
- Timing: Any consistent time daily; rotate injection sites systematically.
Storage Instructions
Proper storage preserves peptide quality and activity.
- Lyophilized: Store at −20 °C (−4 °F) in dry, dark conditions; minimize moisture exposure[7].
- Reconstituted: Refrigerate at 2–8 °C (35.6–46.4 °F); do not freeze reconstituted solution as freezing can denature peptides.
- Allow vials to reach room temperature before opening to reduce condensation uptake.
- Use reconstituted vials within 28 days when stored with bacteriostatic water preservative[8].
Important Notes
Practical considerations for consistency and safety in research protocols.
- Use new sterile insulin syringes for each injection; dispose in a sharps container.
- Rotate injection sites (abdomen, thighs, upper arms) to reduce local irritation and lipohypertrophy[9].
- Inject slowly; wait a few seconds before withdrawing the needle to prevent backflow.
- Document daily dose, injection site, and any observations to maintain consistency.
- Regulatory Note: TB‑500 is banned by WADA for athletic use and is not FDA‑approved for human administration[10].
How This Works
TB‑500 represents the N‑terminal active fragment of thymosin beta‑4, specifically the heptapeptide sequence Ac‑LKKTETQ[1][2]. This region is responsible for the actin‑binding and cell‑migration properties of the full thymosin molecule. Preclinical studies demonstrate that TB‑500 promotes angiogenesis, accelerates wound healing, and supports tissue regeneration by upregulating cell motility and blood vessel formation[5][6]. Research in animal models shows enhanced collagen deposition and reduced healing time in injury sites treated with thymosin fragments[11]. Recent metabolic studies suggest TB‑500 may act as a prodrug, cleaving to an active pentapeptide metabolite that drives biological activity[12].
Potential Benefits & Side Effects
Observations from preclinical and veterinary literature.
- Supports accelerated wound healing and tissue repair through enhanced angiogenesis and cell migration[5][6].
- May reduce inflammation and fibrosis indirectly via thymosin pathways observed in animal models[11].
- Generally well tolerated in veterinary studies; occasional mild injection‑site reactions (redness, tenderness) reported.
- Human safety data is limited; no large‑scale clinical trials have been completed for TB‑500 specifically[13].
Lifestyle Factors
Complementary strategies for optimal research outcomes.
- Maintain adequate protein intake to support tissue repair and regeneration processes.
- Combine with appropriate physical activity; avoid overtraining during injury recovery phases.
- Prioritize sleep (7–9 hours) to maximize natural recovery and repair mechanisms.
- Manage stress levels through evidence‑based practices to support overall healing.
Injection Technique
General subcutaneous guidance from clinical best‑practice resources[14][15].
- Clean the vial stopper and skin with alcohol; allow to air dry completely.
- Pinch a skinfold at the injection site; insert the needle at 45–90° into subcutaneous tissue[14].
- Do not aspirate for subcutaneous injections; inject slowly and steadily[14].
- Rotate sites systematically within approved areas (abdomen, thighs, upper arms) to avoid lipohypertrophy[9].
- Wait 5–10 seconds after injection before withdrawing needle to prevent medication leakage.
Recommended Source
We recommend Pure Lab Peptides for high‑purity TB‑500 (10 mg).
Why Pure Lab Peptides?
- High‑purity peptides (≥99%) with third‑party testing and batch‑specific COAs.
- Consistent quality control and ISO‑aligned handling procedures.
- Reliable fulfillment with proper cold‑chain shipping to maintain peptide integrity.
- Transparent documentation for research compliance.
Important Note
This content is intended for therapeutic educational purposes only and does not constitute medical advice, diagnosis, or treatment. TB‑500 is not approved by the FDA for human use and is for research purposes only. TB‑500 is a prohibited substance in competitive sports under WADA regulations.
References
-
FASEB Journal
— Biological activities of thymosin β4 defined by active peptide sequences (TB‑500 fragment identification) -
Journal of Chromatography A (PubMed)
— Doping control analysis of TB‑500 as synthetic thymosin β4 fragment in biological samples -
WADA Scientific Research
— Investigation of TB‑500 metabolism, synthesis of metabolites, and detection limits -
Racing Medication & Testing Consortium
— Thymosin β4 regulatory bulletin (TB‑500 use in equine sports medicine) -
Journal of Investigative Dermatology (PubMed)
— Thymosin beta4 accelerates wound healing (preclinical wound healing model) -
FASEB Journal (PubMed)
— Active site mapping of thymosin β4 fragments for angiogenesis and cell migration -
Verified Peptides Storage Guide
— Lyophilized peptide storage best practices (temperature, humidity, light protection) -
Empower Pharmacy
— Bacteriostatic water injection guidelines (0.9% benzyl alcohol, multi‑dose vial stability) -
NCBI Bookshelf
— Best practices for subcutaneous injection (aseptic technique, site rotation) -
WADA Prohibited List
— TB‑500 classification as prohibited substance in competitive sports -
Journal of Investigative Dermatology
— Thymosin β4 wound healing mechanisms (collagen deposition, angiogenesis, granulation tissue) -
Journal of Chromatography B (PubMed)
— Quantification of TB‑500 metabolites and wound healing activity screening (prodrug hypothesis) -
CenterWatch Clinical Trials
— Clinical trial registry for thymosin β4 in acute myocardial infarction (no TB‑500 specific trials) -
Nursing Journal (LWW)
— How to administer subcutaneous injections (clinical technique guidelines) -
University of Michigan Health
— Patient education guide for subcutaneous injection technique -
Pure Lab Peptides
— TB‑500 (10 mg) product page (purity specifications and batch documentation)