KLOW (80 mg Vial) Dosage Protocol
Quickstart Highlights
KLOW (80 mg) is a multi‑peptide research blend most commonly labeled as GHK‑Cu 50 mg + BPC‑157 10 mg + TB‑500 10 mg + KPV 10 mg per vial. There is no universally accepted clinical dosing schedule for this blend; this page focuses on precise measurement and reconstitution so researchers can design study‑specific protocols.
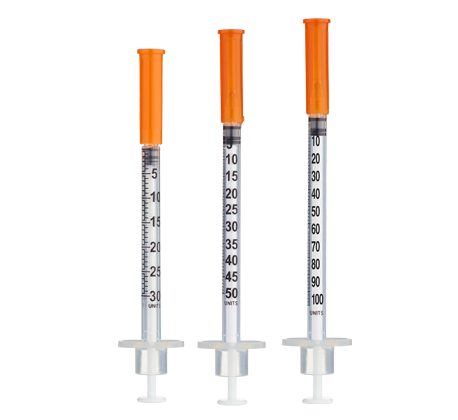
- Reconstitute to a practical concentration and measure with insulin syringes
- At 3 mL reconstitution (max vial capacity), 1 unit (0.01 mL) ≈ 266.7 mcg of total mixture
- Per‑unit component equivalence (3 mL): GHK‑Cu ≈ 166.7 mcg; BPC‑157 ≈ 33.3 mcg; TB‑500 ≈ 33.3 mcg; KPV ≈ 33.3 mcg
- Store lyophilized vials in a cool, dry place; refrigerate after mixing (2–8 °C)
Research use only. Not for human consumption. Not medical advice.

Dosing & Reconstitution Guide
Educational guide for reconstitution and syringe-based measurement (no standardized dosing protocol exists for this blend)
Measurement & Reconstitution (3 mL = ~26,667 mcg/mL mixture)
| Injection Option | Dosage (mixture & per‑component) | Units (mL) |
|---|---|---|
| Option A | ~2.667 mg mixture per injection → GHK‑Cu ~1.667 mg; BPC‑157 ~0.333 mg (333 mcg); TB‑500 ~0.333 mg (333 mcg); KPV ~0.333 mg (333 mcg) | 10 units (0.10 mL) |
| Option B | ~4.000 mg mixture per injection → GHK‑Cu ~2.500 mg; BPC‑157 ~0.500 mg (500 mcg); TB‑500 ~0.500 mg (500 mcg); KPV ~0.500 mg (500 mcg) | 15 units (0.15 mL) |
| Option C | ~5.333 mg mixture per injection → GHK‑Cu ~3.333 mg; BPC‑157 ~0.667 mg (667 mcg); TB‑500 ~0.667 mg (667 mcg); KPV ~0.667 mg (667 mcg) | 20 units (0.20 mL) |
| Option D | ~8.000 mg mixture per injection → GHK‑Cu ~5.000 mg; BPC‑157 ~1.000 mg; TB‑500 ~1.000 mg; KPV ~1.000 mg | 30 units (0.30 mL) |
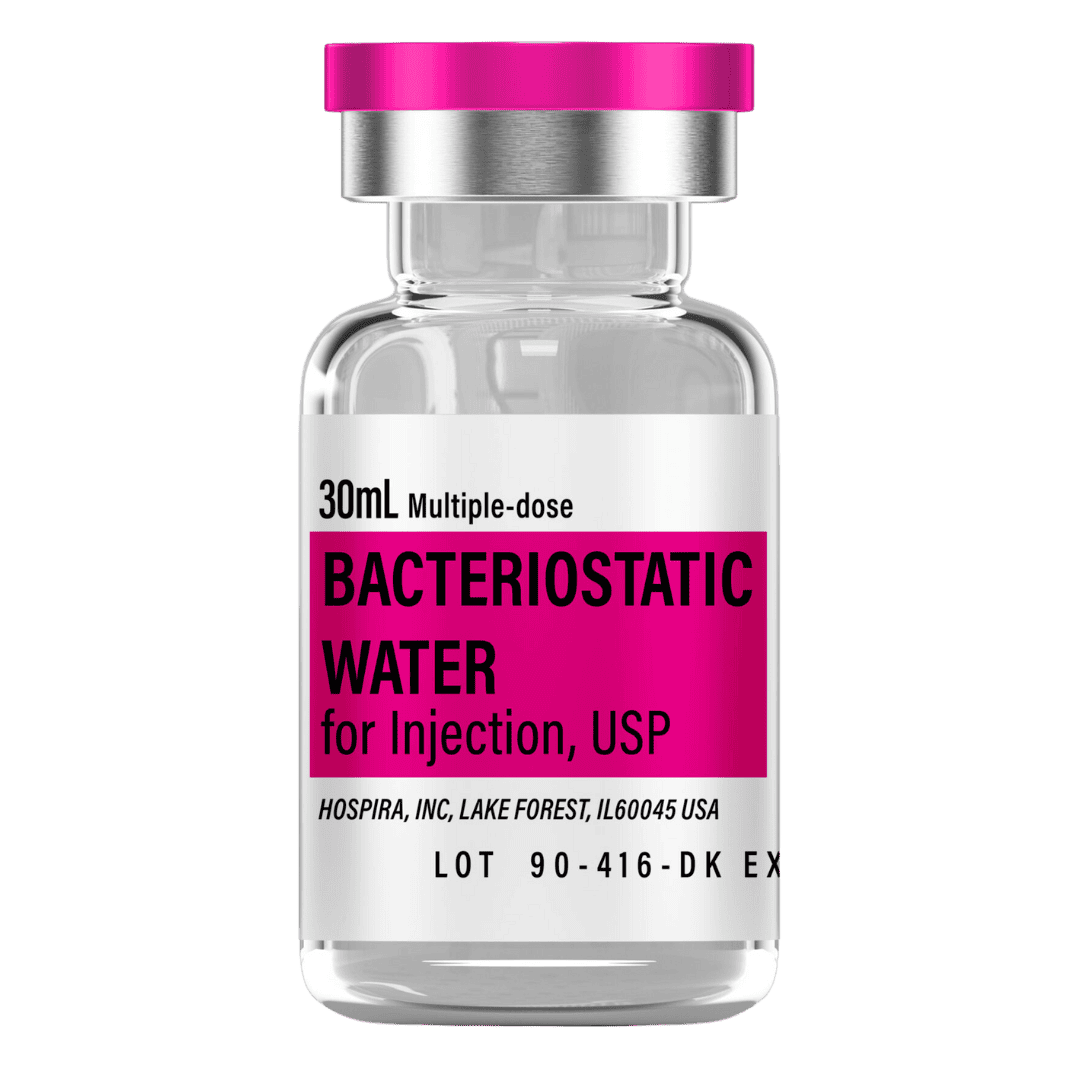
Reconstitute with 3 mL of bacteriostatic water to maximize measurability on 100‑unit insulin syringes (aiming for ≥10 units per injection). If your planned volume would fall below ~10 units at 3 mL dilution, consider smaller insulin syringes (30–50 unit) for precision.
- Draw 3.0 mL of bacteriostatic water into a sterile syringe.
- Inject slowly along the vial wall to minimize foaming; allow powder to dissolve.
- Gently swirl—avoid vigorous shaking.
- Store reconstituted solution at 2–8 °C, protected from light. Do not freeze. Use within ~30 days.
Protocol Overview
A concise summary of reconstitution and per‑unit conversions for this multi‑peptide research blend.
- Blend (typical label): GHK‑Cu 50 mg + BPC‑157 10 mg + TB‑500 10 mg + KPV 10 mg per 80 mg vial
- Reconstitution: 3 mL (max vial capacity) → ~26,667 mcg/mL mixture
- Per‑Unit Equivalence (0.01 mL): ~266.7 mcg mixture; GHK‑Cu ~166.7 mcg; BPC‑157 ~33.3 mcg; TB‑500 ~33.3 mcg; KPV ~33.3 mcg
- Measurement Options: Common increments shown (10u/15u/20u/30u) to keep volumes ≥10 units for accuracy
- Scheduling: Define frequency and total exposure by study design, model, and IACUC/ethics approvals
Dosing Protocol
This page does not prescribe a fixed dosing schedule. Use the conversion table to select volumes appropriate for your protocol endpoints.
- Example increments (per injection): 10u ≈ 2.667 mg mixture; 15u ≈ 4.000 mg; 20u ≈ 5.333 mg
- Frequency: Set per study (e.g., daily, every other day, or weekly), informed by endpoint measures and component literature
- Assay Planning: Record both units and calculated mg/mcg per component for reproducibility
- Injection Route: Typically subcutaneous for solution studies unless model dictates otherwise
- Compliance: Research use only; adhere to local/institutional regulations
Storage Instructions
Proper storage supports peptide integrity.
- Lyophilized: Cool, dry, light‑protected storage
- Reconstituted: Refrigerate at 2–8 °C; avoid repeated freeze–thaw
- Use Within: ~30 days after mixing (laboratory best practice)
- Handling: Aseptic technique; label vial with date, diluent, and concentration
Supplies Needed
Plan supplies based on your study’s injection frequency and chosen volume.
- Peptide Vials (Product ID: 17302):
Capacity per vial @ 3 mL:
• 10u per injection → ~30 injections/vial
• 15u per injection → ~20 injections/vial
• 20u per injection → ~15 injections/vial
• 30u per injection → ~10 injections/vial
Example planning: 2 injections/week at 20u ≈ ~7–8 weeks per vial; adjust to your schedule. - Insulin Syringes: 100‑unit; consider 30–50‑unit syringes for finer measurements
- Bacteriostatic Water: 1× 30 mL
- Alcohol Swabs: 1 box
Important Notes
Practical tips to enhance safety and data quality.
- Use sterile technique; rotate injection sites; document lot numbers and dates
- Pre‑calculate component mg/mcg delivered per injection for transparent reporting
- Monitor the model for adverse responses; halt per IACUC/ethics protocol if concerns arise
- Ensure chain‑of‑custody and cold‑chain integrity for reconstituted solutions
How This Works
KLOW combines four peptides studied across complementary pathways relevant to tissue modeling and inflammation.
- GHK‑Cu: Copper‑binding tripeptide associated with ECM remodeling, collagen/elastin synthesis, and pro‑repair gene expression; evaluated in in vitro, animal, and dermatologic studies.
- TB‑500 (Thymosin β4 fragment): Linked to cell migration (actin dynamics), angiogenesis, and wound repair; Tβ4 formulations (e.g., RGN‑137) have been investigated clinically for dermal healing and corneal injury.
- BPC‑157: Gastric‑derived pentadecapeptide reported to modulate angiogenesis and NO signaling with cytoprotective effects in numerous animal models; limited early human safety/PK data.
- KPV (α‑MSH 11‑13): C‑terminal tripeptide of α‑MSH with anti‑inflammatory activity via melanocortin‑related pathways and PepT1‑mediated uptake; studied in colitis and mucosal healing models.
Potential Research Signals & Considerations
Preclinical literature suggests roles in ECM remodeling, angiogenesis, and inflammation modulation; translational significance remains under active investigation.
- Observed signals in models: increased collagen deposition, enhanced cell migration and re‑epithelialization, reduced inflammatory markers
- Potential adverse events (general to peptide studies): injection‑site reactions, edema, headache or fatigue; long‑term safety of combinations is not established
- Follow institutional and regulatory guidance; verify each component against applicable competition or compliance rules
Study Design Factors
Simple controls that can strengthen outcomes.
- Standardize timing (circadian) and route of administration
- Control diet, hydration, and physical activity across cohorts where relevant
- Predefine endpoints (histology, biomechanical testing, cytokine panels) and blinding
Injection Technique
General laboratory guidance for subcutaneous administration.
- Disinfect stopper and site with alcohol swabs; use new sterile needle/syringe each time
- Pinch subcutaneous tissue; insert at 45–90°; inject slowly
- Rotate sites (abdomen, thigh) and document volumes/locations
Recommended Source
We recommend Pure Lab Peptides for high‑purity research peptides (e.g., GHK‑Cu, BPC‑157, TB‑500, KPV).
Why Pure Lab Peptides?
- Independent lab verification with typical ≥99% purity
- ISO‑aligned quality controls for consistent research results
- Transparent COAs and batch traceability
All products are sold for laboratory research use only.
Important Note
This guide is for educational and research purposes only. It does not provide medical advice and is not a treatment recommendation.
References
-
-
Maquart et al., FEBS Lett.
– GHK‑Cu stimulates collagen synthesis in fibroblasts -
Pickart & Margolina, J Aging Res Clin Pract / Reviews
– GHK/ GHK‑Cu biology and skin remodeling overview -
Pickart et al., Dermatology/Cosmetics Review
– Regenerative & protective actions of GHK‑Cu (gene data) -
JAMA Facial Plast. Surg.
– Topical copper tripeptide complex in CO₂ laser resurfacing patients
-
-
-
Zhang et al., Front. Pharmacol. (Review)
– Progress on function & application of Thymosin β4 -
Kaur et al., J Invest Dermatol / Reviews
– Tβ4 as a multi‑functional regenerative peptide -
NCT00832091 – ClinicalTrials.gov
– Tβ4 (RGN‑137) in venous stasis ulcers (dermal wound trial) -
Sosne et al., Ocul. Surf. / PMC
– Thymosin β4 as corneal wound‑healing and anti‑inflammatory agent
-
-
-
Seiwerth et al., Front. Pharmacol. 2021
– BPC‑157 and wound healing mechanisms (review) -
Šikić et al., Pharmaceuticals 2024
– Pleiotropic effects and limited early human evidence (review) -
Chang et al., J Appl Physiol. 2011
– BPC‑157 promotes tendon/ligament healing in rodent models -
NCT02637284 – ClinicalTrials.gov
– Phase I safety & PK of BPC‑157 in healthy volunteers -
Lee et al., PubMed 2025
– Pilot: IV BPC‑157 up to 20 mg in two adults (tolerability) -
Vasireddi et al., Orthop. Sports Med. 2025
– Systematic review of BPC‑157 in musculoskeletal models
-
-
-
Catania et al., Br J Pharmacol. (Review)
– α‑MSH peptides as anti‑inflammatory agents; KPV as minimal active sequence -
Dalmasso et al., Gastroenterology
– PepT1‑mediated uptake of KPV reduces colitis -
Kannengiesser et al., Inflamm Bowel Dis.
– KPV anti‑inflammatory effects in murine colitis models -
Xiao et al., Mol Ther. 2017
– HA‑targeted nanoparticles delivering KPV for ulcerative colitis
-
-
Vendor Label – KLOW (80 mg) Spec
– GHK‑Cu 50 mg / BPC‑157 10 mg / TB‑500 10 mg / KPV 10 mg per vial -
Vendor Label – KLOW (80 mg) Spec
– Blend listing across suppliers (GHK‑Cu/BPC‑157/TB‑500/KPV)