L-Carnitine (200 mg Vial) Dosage Protocol
Quickstart Highlights
L-Carnitine is an amino acid derivative essential for fatty acid transport into mitochondria, where it facilitates β-oxidation and energy production[1]. Subcutaneous administration bypasses intestinal conversion to trimethylamine-N-oxide (TMAO), a metabolite associated with cardiovascular concerns[4], while providing 100% bioavailability compared to 5–18% for large oral doses[3]. This educational protocol presents a once-daily subcutaneous approach optimized for insulin-syringe measurements.
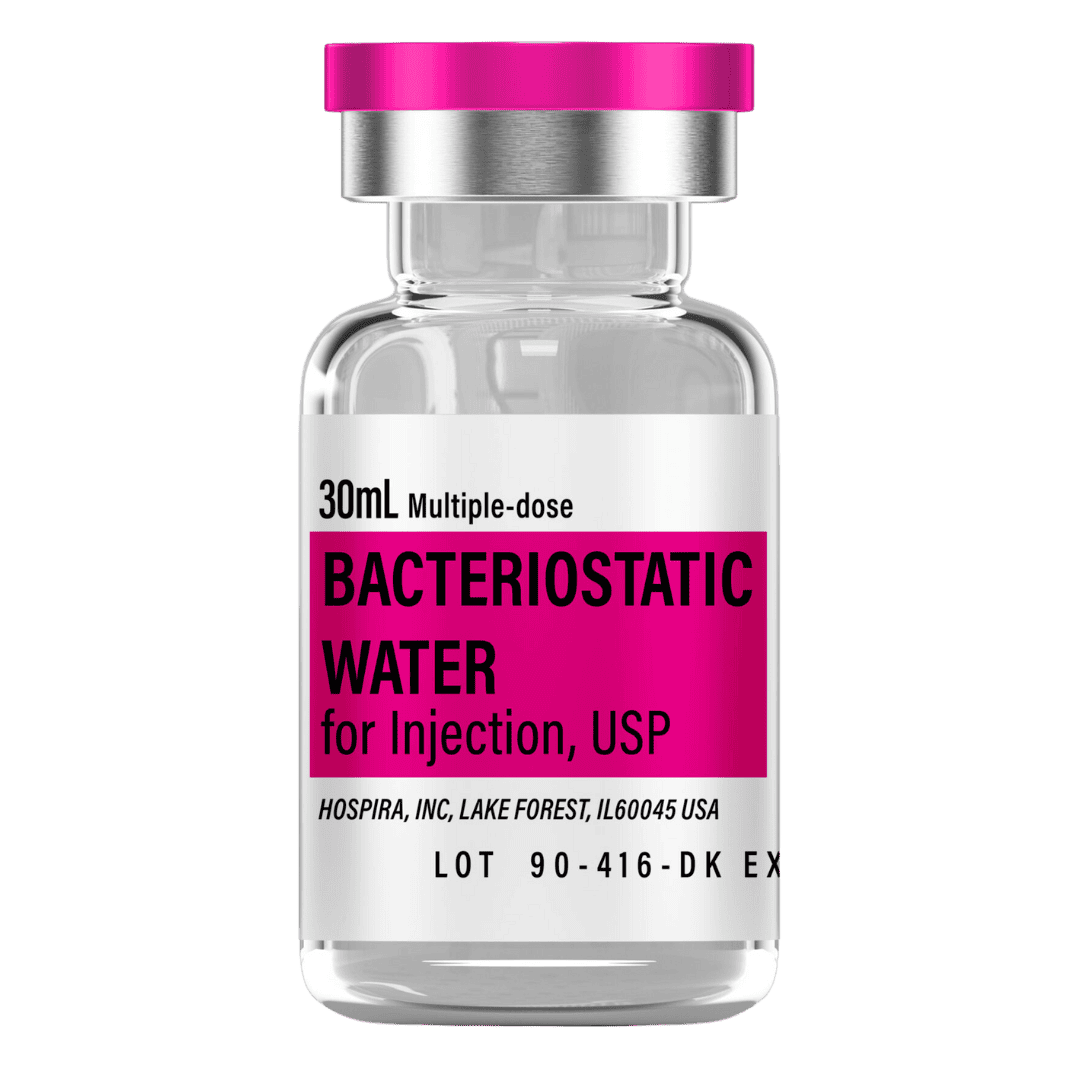
- Reconstitute: Add 2.0 mL bacteriostatic water → 100 mg/mL concentration (1 unit = 1 mg).
- Typical daily range: 50–100 mg once daily (gradual titration); advanced protocols may use up to 200 mg.
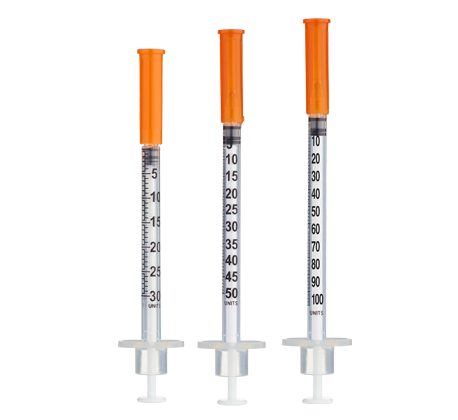
- Easy measuring: At 100 mg/mL, 1 unit = 0.01 mL = 1 mg on a U-100 insulin syringe.
- Storage: Lyophilized: freeze at −20 °C (−4 °F); after reconstitution, refrigerate at 2–8 °C (35.6–46.4 °F) and use within 2–4 weeks; do not freeze reconstituted solution.

Dosing & Reconstitution Guide
Educational guide for reconstitution and daily dosing
Standard / Gradual Approach (2.0 mL = 100 mg/mL)
| Week | Daily Dose | Units (per injection) (mL) |
|---|---|---|
| Weeks 1–2 | 50 mg | 50 units (0.50 mL) |
| Weeks 3–8 | 100 mg | 100 units (1.0 mL) |
| Weeks 9–12 | 100 mg | 100 units (1.0 mL) |
Frequency: Inject once daily subcutaneously. This 2.0 mL reconstitution provides optimal concentration for convenient dosing: 100 mg maintenance dose = exactly 1.0 mL (100 units)[8]. For advanced protocols requiring 150–200 mg daily, doses above 1.0 mL may be split into two injections at different sites to improve comfort.
Reconstitution Steps
- Draw 2.0 mL bacteriostatic water (0.9% benzyl alcohol) with a sterile syringe.
- Inject slowly down the vial wall to minimize foaming; avoid direct stream onto powder.
- Gently swirl or roll the vial until powder is fully dissolved (do not shake vigorously).
- Label vial with reconstitution date and refrigerate at 2–8 °C (35.6–46.4 °F), protected from light.
- Use within 2–4 weeks; bacteriostatic water preservative inhibits microbial growth during multi-dose use[6].
Supplies Needed
Plan based on an 8–16 week daily protocol using 100 mg maintenance dose (most common). If starting with 50 mg titration (Weeks 1–2), subtract ~3–4 vials from totals below.
-
Peptide Vials (L-Carnitine, 200 mg each):
- 8 weeks (56 days @ 100 mg/day) ≈ 28 vials
- 12 weeks (84 days @ 100 mg/day) ≈ 42 vials
- 16 weeks (112 days @ 100 mg/day) ≈ 56 vials
Note: Each 200 mg vial reconstituted at 2.0 mL provides two 100 mg doses (or four 50 mg doses).
-
Insulin Syringes (U-100, 1 mL capacity):
- Per week: 7 syringes (1/day)
- 8 weeks: 56 syringes
- 12 weeks: 84 syringes
- 16 weeks: 112 syringes
-
Bacteriostatic Water (10 mL bottles): Use 2.0 mL per vial for reconstitution.
- 8 weeks (28 vials): 56 mL → 6 × 10 mL bottles
- 12 weeks (42 vials): 84 mL → 9 × 10 mL bottles
- 16 weeks (56 vials): 112 mL → 12 × 10 mL bottles
-
Alcohol Swabs: One for vial stopper + one for injection site each day.
- Per week: 14 swabs (2/day)
- 8 weeks: 112 swabs → recommend 2 × 100-count boxes
- 12 weeks: 168 swabs → recommend 2 × 100-count boxes
- 16 weeks: 224 swabs → recommend 3 × 100-count boxes
- Sharps Container: For safe disposal of used syringes and needles.
Protocol Overview
Concise summary of the once-daily subcutaneous regimen.
- Goal: Support mitochondrial fatty acid oxidation and energy metabolism while avoiding TMAO production associated with oral dosing[4].
- Schedule: Daily subcutaneous injections for 8–12 weeks (extend to 16 weeks if desired).
- Dose Range: 50–100 mg daily with gradual titration; advanced protocols may use up to 200 mg daily.
- Reconstitution: 2.0 mL per 200 mg vial (100 mg/mL) for precise 1:1 unit-to-milligram measurement.
- Storage: Lyophilized frozen; reconstituted refrigerated; avoid repeated freeze–thaw cycles.
Dosing Protocol
Suggested daily titration approach based on clinical literature.
- Start: 50 mg daily for Weeks 1–2 to assess tolerance and monitor for injection-site reactions[8].
- Maintenance: Increase to 100 mg daily from Week 3 onward; this is a reasonable maintenance dose for most research purposes[8].
- Advanced: Up to 200 mg daily for robust experimental protocols, if clearly supported by research findings[8].
- Frequency: Once per day subcutaneously (any consistent time; morning or pre-exercise commonly used).
- Cycle Length: 8–12 weeks; optional extension to 16 weeks with continued monitoring.
- Site Rotation: Rotate injection sites systematically (abdomen, thighs, upper arms) to prevent tissue irritation.
Storage Instructions
Proper storage preserves peptide stability and potency.
- Lyophilized (unreconstituted): Store at −20 °C (−4 °F) or below in dry, dark conditions; minimize moisture exposure. Keep vial tightly sealed. Stable for months to years when frozen[13].
- Reconstituted solution: Refrigerate at 2–8 °C (35.6–46.4 °F) immediately after mixing. Do not freeze reconstituted solution as this can degrade the peptide[8].
- Stability timeline: With bacteriostatic water (0.9% benzyl alcohol), reconstituted L-Carnitine remains usable for 2–4 weeks when refrigerated[8][13]. Benzyl alcohol suppresses bacterial growth during multi-dose use[6].
- Before use: Allow refrigerated vials to reach room temperature before opening to reduce condensation. Inspect solution before each use; discard if discolored or contains precipitates.
- Avoid freeze–thaw cycles: Repeated freezing and thawing degrades peptide integrity; aliquot into smaller vials if long-term storage is needed[13].
Important Notes
Practical considerations for consistency, safety, and optimal results.
- Sterile technique: Use new sterile insulin syringes for each injection; never reuse needles or syringes.
- Site rotation: Rotate injection sites systematically (abdomen at least 2 inches from navel, outer thighs, upper arms) to reduce local irritation and prevent lipohypertrophy[11].
- Injection speed: Inject slowly (over several seconds) to minimize discomfort; wait 5–10 seconds before withdrawing needle to prevent leakage[6].
- Room temperature: Allow refrigerated solution to warm slightly (to room temperature) before injecting to reduce pain[6].
- Documentation: Keep a log of daily dose, injection site, and any observations (tolerance, energy levels) to maintain consistency.
- Sharps disposal: Immediately dispose of used needles and syringes in an approved sharps container; never recap needles by hand.
How This Works
L-Carnitine is a quaternary ammonium compound biosynthesized from lysine and methionine that plays a critical role in energy metabolism. It acts as an obligate cofactor for the carnitine palmitoyltransferase (CPT) enzyme system, which shuttles long-chain fatty acids across the inner mitochondrial membrane for β-oxidation[1]. This process is essential for ATP production from fat stores, particularly during prolonged exercise or caloric restriction.
Clinical and preclinical studies indicate L-Carnitine supplementation can enhance fat oxidation, reduce body weight, and improve exercise performance in certain populations[2][5]. A meta-analysis of 37 randomized controlled trials found approximately 2,000 mg/day oral L-Carnitine yields modest weight-loss effects (~1.2 kg), with diminishing returns above that dose[2].
Why subcutaneous over oral? Oral L-Carnitine has poor bioavailability (5–18% at high doses) due to saturable intestinal absorption[3]. Furthermore, unabsorbed carnitine is metabolized by gut bacteria into trimethylamine (TMA), which is converted to trimethylamine-N-oxide (TMAO) in the liver—a metabolite linked to increased cardiovascular risk[4][9]. Subcutaneous or intravenous administration provides 100% bioavailability and bypasses TMAO production, as demonstrated in animal models where parenteral L-Carnitine did not promote atherosclerosis unlike oral dosing[4].
In hemodialysis patients with carnitine deficiency, intravenous L-Carnitine (10–40 mg/kg after dialysis sessions) significantly increased plasma carnitine levels, reduced fatigue, and preserved exercise capacity over 24 weeks with excellent tolerability[5]. High-dose intravenous protocols (up to 50 mg/kg daily, ~3,500 mg for a 70 kg person) have been used safely in patients with metabolic disorders[8], indicating a wide therapeutic margin.
Potential Benefits & Side Effects
Observations from clinical and preclinical literature on L-Carnitine supplementation.
Potential Benefits
- Enhanced fat metabolism: Facilitates mitochondrial fatty acid oxidation and ATP production from lipid stores[1].
- Weight management: Meta-analysis shows modest weight loss (~1.2 kg average) and reduction in BMI with oral supplementation at ~2 g/day[2].
- Exercise performance: May improve exercise capacity and reduce fatigue, particularly in carnitine-deficient populations[5].
- Avoids TMAO production: Subcutaneous route bypasses gut bacterial conversion to TMAO, potentially reducing cardiovascular risk associated with oral dosing[4][9].
- Energy support: Clinical studies in dialysis patients show reduced fatigue and improved well-being with parenteral L-Carnitine[5].
Potential Side Effects
- Injection-site reactions: Mild redness, swelling, or irritation at injection sites (typically transient). Rotate sites systematically to minimize[6].
- Gastrointestinal effects (oral): High oral doses (>3 g/day) can cause nausea, diarrhea, and fishy body odor from metabolite accumulation[9]. Subcutaneous administration minimizes these effects by reducing required dose and avoiding first-pass gut metabolism[8].
- Generally well-tolerated: Clinical trials report good safety profiles with parenteral L-Carnitine, even at high doses (up to 50 mg/kg IV daily)[5][8].
Note: This information is for educational purposes only. Individual responses may vary. Consult appropriate resources before beginning any supplementation protocol.
Lifestyle Factors
Complementary strategies to optimize L-Carnitine’s metabolic effects.
- Balanced nutrition: Pair with a protein-forward diet (1.6–2.2 g/kg bodyweight) to support lean mass retention during fat loss. Ensure adequate essential amino acids (lysine and methionine) for endogenous carnitine synthesis[1].
- Exercise synergy: Combine resistance training (3–5×/week) with aerobic activity (150+ min/week moderate intensity) to maximize fat oxidation and metabolic adaptations. L-Carnitine’s effects on fat metabolism are enhanced during exercise[2].
- Caloric management: Create a modest caloric deficit (300–500 kcal/day) if fat loss is the goal. L-Carnitine does not override energy balance but may enhance fat utilization within a deficit[2].
- Sleep optimization: Prioritize 7–9 hours of quality sleep nightly to support recovery, hormonal balance, and adherence to training/nutrition protocols.
- Hydration: Maintain adequate fluid intake (minimum 2–3 liters/day, more with exercise) to support metabolic processes and overall health.
- Stress management: Chronic stress elevates cortisol, which can impair fat loss and recovery. Incorporate stress-reduction practices (meditation, breathing exercises, adequate rest).
Injection Technique
Proper subcutaneous injection technique based on CDC guidelines and clinical best practices[11][12].
Preparation
- Hand hygiene: Wash hands thoroughly with soap and water or use alcohol-based hand sanitizer.
- Materials: Gather sterile U-100 insulin syringe (1 mL capacity, 25–30 gauge needle), alcohol swabs, sharps container, and refrigerated L-Carnitine vial.
- Warm vial: Allow vial to reach room temperature (or roll gently between palms) to reduce injection discomfort[6].
- Vial prep: Swab rubber stopper with alcohol and allow to dry (~30 seconds).
- Draw dose: Insert needle through stopper, draw prescribed volume (e.g., 100 units = 1.0 mL for 100 mg dose), and expel any air bubbles by tapping syringe and pushing plunger slightly.
Injection Procedure
- Site selection: Choose a fatty subcutaneous area. Common sites include[11]:
- Abdomen (at least 2 inches away from navel)
- Outer thigh (middle third, avoiding inner thigh)
- Upper outer arm (triceps region, if sufficient subcutaneous tissue)
- Site rotation: Keep a rotation log to avoid using the same site within 1–2 weeks. This prevents lipohypertrophy and tissue damage[11].
- Skin antisepsis: Clean injection site with alcohol swab using outward circular motion; allow to dry completely (~30 seconds).
- Needle insertion: Pinch a fold of skin (1–2 inches) between thumb and forefinger. Insert needle at a 45° angle (or 90° if using very short needle and ample subcutaneous tissue) into the subcutaneous fat layer[11][12].
- No aspiration needed: Aspiration (pulling back on plunger) is not necessary for subcutaneous injections, as there are no large blood vessels in subcutaneous tissue[11].
- Inject slowly: Depress plunger steadily over 3–5 seconds to minimize discomfort. Injecting volumes <1.0 mL causes minimal pain; larger volumes (1.5–2.0 mL) may be split into two separate injections[6].
- Wait before withdrawal: After full injection, wait 5–10 seconds before withdrawing needle to prevent solution leakage.
- Needle removal: Withdraw needle at the same angle as insertion. Release skin pinch.
Post-Injection
- Pressure/bleeding: Apply gentle pressure with clean gauze or cotton ball if slight bleeding occurs (uncommon). Do not rub the injection site vigorously.
- Sharps disposal: Immediately place used syringe and needle into an approved sharps container. Never recap needles or dispose in regular trash[12].
- Documentation: Record date, time, dose, and injection site in your protocol log.
Tips for Comfort
- Inject solution at room temperature (not cold from refrigerator) to reduce pain[6].
- Use fresh needles for each injection; dull or reused needles increase pain and infection risk.
- Relax muscles at injection site; tension increases discomfort.
- Benzyl alcohol in bacteriostatic water causes less injection pain than other preservatives (e.g., m-cresol)[6].
Recommended Source
We recommend Pure Lab Peptides for high-purity L-Carnitine (200 mg) with comprehensive quality documentation.
Why Pure Lab Peptides?
- Third-party testing: Each batch includes Certificates of Analysis (COA) confirming purity ≥98% via HPLC and mass spectrometry.
- Quality assurance: ISO-aligned handling, sterile manufacturing, and consistent cold-chain logistics ensure product integrity.
- Transparent documentation: Batch-specific COAs, proper labeling, and reconstitution guidance provided with every order.
- Reliable fulfillment: Fast shipping with appropriate cold packs/insulation to maintain stability during transit.
- Research-grade: Manufactured specifically for laboratory research purposes with strict quality controls.
Important Note
This content is intended for therapeutic educational purposes only and does not constitute medical advice, diagnosis, or treatment. L-Carnitine is provided for research purposes only and is not intended for human consumption. All protocols described are for educational and informational purposes. Consult qualified professionals before beginning any supplementation or research protocol.
References
-
Journal of the International Society of Sports Nutrition (2020)
— Sawicka AK et al. The bright and the dark sides of L-carnitine supplementation: a systematic review (dose ranges, mechanisms, and metabolic effects) -
Clinical Nutrition ESPEN (2020)
— Talenezhad N et al. Meta-analysis of 37 RCTs: L-carnitine supplementation yields ~1.2 kg weight loss at ~2,000 mg/day oral dosing with diminishing returns above that dose (PMID: 32359762) -
Clinical Pharmacokinetics (2003)
— Evans AM & Fornasini G. Pharmacokinetics of L-carnitine: oral bioavailability only 5–18% at high doses (1–6 g) vs. much higher absorption from IV/parenteral routes (PMID: 12908852) -
Molecular Nutrition & Food Research (2018)
— Zhao Y et al. Subcutaneous L-carnitine bypasses gut bacterial TMAO production and does not promote atherosclerosis in ApoE−/− mice, unlike oral dosing (PMID: 29178259) -
American Journal of Kidney Diseases (2001)
— Brass EP et al. IV L-carnitine (10–40 mg/kg after dialysis, 3×/week for 24 weeks) increased plasma carnitine, reduced fatigue, preserved exercise capacity in hemodialysis patients with excellent safety profile (PMID: 11325685) -
Advances in Therapy (2019)
— Usach I et al. Subcutaneous injection review: volumes <0.8 mL minimize pain; room-temperature solutions, benzyl alcohol (vs. m-cresol) reduce pain; abdomen preferred site (PMID: 31529256) -
CDC Pink Book (14th Edition)
— Vaccine Administration (Chapter 6): subcutaneous injection technique (45–90° angle, no aspiration needed, site selection/rotation) -
Drugs.com (2024)
— Levocarnitine (Carnitor) dosing: 50 mg/kg IV daily for metabolic disorders; 10–20 mg/kg IV after dialysis for ESRD; doses up to 300 mg/kg/day used safely (medically reviewed Apr 23, 2024) -
NIH Office of Dietary Supplements (2022)
— Carnitine Health Professional Fact Sheet: high oral doses (>3 g/day) cause fishy odor, nausea; unabsorbed carnitine → gut bacterial TMAO production (potential CV risk) -
Linus Pauling Institute, Oregon State University (2022)
— L-Carnitine Micronutrient Information Center: FDA-approved uses (dialysis 10–20 mg/kg IV), research summary, safety overview -
CDC Vaccine Administration Guidelines
— Best practices during vaccination: subcutaneous injection angle (45–90°), site selection, no aspiration needed, rotation to prevent lipohypertrophy -
NCBI Bookshelf
— Clinical Procedures: Best Practices in Injection Administration (aseptic technique, preparation, sharps disposal) -
SB Peptide
— Peptide Handling & Storage Guidelines: lyophilized peptides stable for months–years at −20 °C; reconstituted solutions stable 1–2 weeks at 4 °C, longer if frozen in aliquots; avoid freeze–thaw cycles -
Pure Lab Peptides
— L-Carnitine (200 mg) Product Page: high-purity research peptide with third-party COA documentation