SS-31 (10 mg Vial) Dosage Protocol
Quickstart Highlights
SS‑31 (elamipretide) is a mitochondria‑targeted tetrapeptide that selectively binds cardiolipin in the inner mitochondrial membrane[1], stabilizing electron transport chain complexes and reducing reactive oxygen species production while enhancing ATP synthesis[2]. This peptide has demonstrated protective effects in preclinical models of heart failure, neurodegenerative disease, and age‑related muscle atrophy, and received FDA accelerated approval in 2025 as the first treatment for Barth syndrome[3]. This educational protocol presents a once‑daily subcutaneous approach using concentrated reconstitution for practical insulin‑syringe measurements.
- Reconstitute: Add 1.0 mL bacteriostatic water → 10 mg/mL concentration for single‑syringe convenience.
- Typical daily range: 5–10 mg once daily (gradual titration); advanced protocols may reach 15–20 mg/day under supervision.
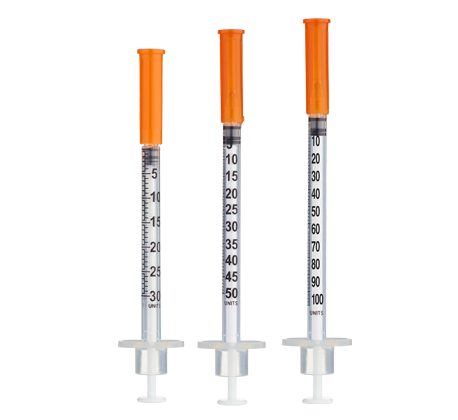
- Easy measuring: At 10 mg/mL, 1 unit = 0.01 mL = 100 mcg on a U‑100 insulin syringe.
- Storage: Lyophilized: freeze at −20 °C (−4 °F); after reconstitution, refrigerate at 2–8 °C (35.6–46.4 °F); use within 4 weeks.

Dosing & Reconstitution Guide
Educational guide for reconstitution and daily dosing
Standard / Gradual Approach (1 mL = 10 mg/mL)
| Week | Daily Dose (mg) | Units (per injection) (mL) |
|---|---|---|
| Weeks 1–2 | 5 mg (5000 mcg) | 50 units (0.50 mL) |
| Weeks 3–8 | 10 mg (10,000 mcg) | 100 units (1.0 mL) |
Frequency: Inject once daily subcutaneously at a consistent time. This concentrated dilution (1.0 mL per 10 mg vial) keeps standard doses within a single insulin syringe for convenience and accuracy.
Reconstitution Steps
- Draw 1.0 mL bacteriostatic water with a sterile syringe.
- Inject slowly down the vial wall; avoid vigorous shaking to prevent foaming.
- Gently swirl/roll until fully dissolved (clear solution).
- Label with reconstitution date and refrigerate at 2–8 °C (35.6–46.4 °F), protected from light; use within 4 weeks.
Advanced / Aggressive Approach (1 mL = 10 mg/mL)
| Week | Daily Dose (mg) | Units (per injection) (mL) |
|---|---|---|
| Weeks 1–2 | 5 mg (5000 mcg) | 50 units (0.50 mL) |
| Weeks 3–4 | 10 mg (10,000 mcg) | 100 units (1.0 mL) |
| Weeks 5–8 | 15 mg (15,000 mcg) | Split: 2 × 75 units (0.75 mL each) |
| Optional Weeks 9–12 | 20 mg (20,000 mcg) | Split: 2 × 100 units (1.0 mL each) |
Note: Advanced dosing (15–20 mg/day) is based on short‑term clinical trial protocols[4][5] for severe mitochondrial conditions and should only be pursued under medical supervision. Doses above 10 mg require splitting into two separate subcutaneous injections at different sites. Clinical trials have not extensively evaluated SS‑31 beyond 12 weeks; extended use requires careful monitoring.
Supplies Needed
Plan based on an 8–12 week daily protocol with gradual titration.
-
Peptide Vials (SS‑31, 10 mg each):
- 8 weeks (Standard: 5–10 mg/day) ≈ 50 vials
- 12 weeks (Standard maintenance at 10 mg/day) ≈ 77 vials
- 12 weeks (Advanced: escalating to 15 mg/day) ≈ 105 vials
-
Insulin Syringes (U‑100, 1 mL capacity):
- Per week (standard dosing): 7 syringes (1/day)
- 8 weeks: 56 syringes
- 12 weeks: 84 syringes
- Advanced protocols with split injections may require 2 syringes per day for higher doses
-
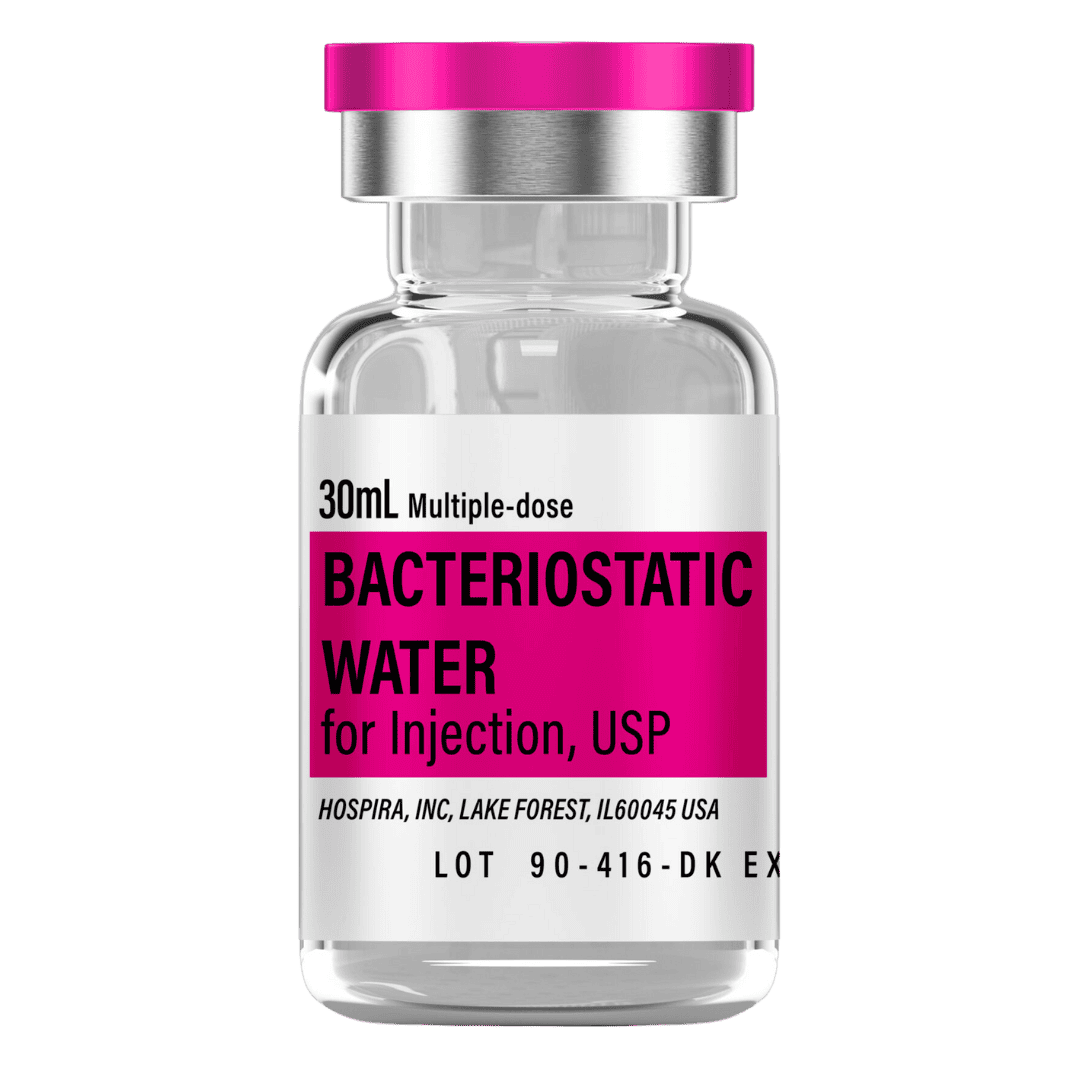
Bacteriostatic Water (30 mL bottles): Use 1.0 mL per vial for reconstitution.
- 8 weeks (50 vials): 50 mL → 2 × 30 mL bottles
- 12 weeks (77 vials): 77 mL → 3 × 30 mL bottles
- 12 weeks (105 vials, advanced): 105 mL → 4 × 30 mL bottles
-
Alcohol Swabs: One for the vial stopper + one for the injection site each day.
- Per week: 14 swabs (2/day)
- 8 weeks: 112 swabs → recommend 2 × 100‑count boxes
- 12 weeks: 168 swabs → recommend 2 × 100‑count boxes
Protocol Overview
Concise summary of the once‑daily subcutaneous regimen.
- Goal: Support mitochondrial function, enhance ATP production, and reduce oxidative stress in tissues with high metabolic demand[1][2].
- Schedule: Daily subcutaneous injections for 8–12 weeks (clinical trials typically 4–12 weeks).
- Dose Range: 5–10 mg daily (standard); 15–20 mg daily (advanced, under supervision).
- Reconstitution: 1.0 mL per 10 mg vial (10 mg/mL) for convenient single‑syringe administration.
- Storage: Lyophilized frozen at −20 °C (−4 °F); reconstituted refrigerated at 2–8 °C (35.6–46.4 °F); use within 4 weeks.
Dosing Protocol
Suggested daily titration approach based on clinical trial protocols[4][5].
- Start: 5 mg daily for Weeks 1–2 to assess individual tolerance.
- Standard Target: 10 mg daily by Week 3 (most common clinical trial dose).
- Advanced Target: 15–20 mg daily for Weeks 5+ (only for severe conditions under medical oversight).
- Frequency: Once per day (subcutaneous injection); maintain consistent timing.
- Cycle Length: 8–12 weeks; limited data exists beyond 12 weeks.
- Timing: Any consistent time; rotate injection sites to prevent tissue irritation.
Storage Instructions
Proper storage preserves peptide stability and potency.
- Lyophilized: Store at −20 °C (−4 °F) in original sealed vial; keep dry and protected from light. Stable for months when frozen.
- Reconstituted: Refrigerate at 2–8 °C (35.6–46.4 °F) immediately after mixing; use within 4 weeks for optimal stability.
- Avoid freeze–thaw: Do not refreeze reconstituted solution; prepare aliquots if needed to minimize temperature fluctuations.
- Allow refrigerated vials to reach room temperature before opening to reduce condensation inside the vial.
Important Notes
Practical considerations for consistency, safety, and tolerability.
- Use new sterile insulin syringes for each injection; dispose in a sharps container immediately after use.
- Rotate injection sites systematically (abdomen, thighs, upper arms) to reduce local irritation and prevent lipohypertrophy[14].
- Inject slowly over several seconds; wait briefly before withdrawing the needle to prevent leakage.
- Mild injection‑site reactions (redness, itching, transient discomfort) are the most common side effects reported in clinical trials[4][6]; these typically resolve within hours.
- Document daily dose, injection site, and any adverse reactions to maintain consistency and identify patterns.
- For doses requiring split injections (≥15 mg), space injections at least 2 inches apart on different body regions.
How This Works
SS‑31 (elamipretide) is a cell‑permeable tetrapeptide with a unique mechanism of action targeting mitochondrial dysfunction[1]. The peptide selectively accumulates in the inner mitochondrial membrane where it binds to cardiolipin, a specialized phospholipid essential for organizing electron transport chain supercomplexes and maintaining cristae structure[2][7]. By stabilizing cardiolipin‑protein interactions, SS‑31 optimizes electron transport efficiency, reduces pathological reactive oxygen species generation, and enhances ATP synthesis in metabolically active tissues[8].
Preclinical research demonstrated that SS‑31 protects against mitochondrial dysfunction across multiple disease models including heart failure, ischemia‑reperfusion injury, neurodegeneration, chronic kidney disease, and age‑related muscle atrophy[9]. In human clinical trials, SS‑31 showed favorable safety and tolerability profiles with no dose‑limiting toxicities[4][5]. While Phase II trials in heart failure and primary mitochondrial myopathy did not meet primary efficacy endpoints, the TAZPOWER trial in Barth syndrome demonstrated significant improvements in muscle strength and six‑minute walk distance, leading to FDA accelerated approval in 2025[3][10].
Potential Benefits & Side Effects
Observations from preclinical models and human clinical trials.
Potential Benefits:
- Stabilizes mitochondrial membranes and optimizes electron transport chain function[1][2].
- Enhances ATP production and reduces pathological reactive oxygen species in metabolically active tissues[8].
- Demonstrated functional improvements in Barth syndrome patients (increased muscle strength, improved exercise capacity)[3][10].
- Shows protective effects in preclinical models of heart failure, neurodegenerative disease, and age‑related muscle atrophy[9].
- Does not significantly alter blood pressure, heart rate, or standard laboratory parameters in clinical trials[4].
Common Side Effects:
- Injection‑site reactions (most common): Mild‑to‑moderate redness, itching, or transient discomfort at injection site; typically resolves within hours[4][6].
- Approximately 80% of patients in clinical trials experienced mild injection‑site reactions[6].
- No dose‑limiting toxicities or serious adverse events directly attributed to SS‑31 in published trials[4][5].
- Long‑term safety data beyond 12 weeks remains limited; extended use requires monitoring.
Lifestyle Factors
Complementary strategies to support mitochondrial health and optimize outcomes.
- Nutrition: Emphasize mitochondrial cofactors including B‑vitamins, CoQ10, magnesium, and alpha‑lipoic acid; maintain adequate protein intake to support muscle maintenance.
- Exercise: Combine resistance training with aerobic activity to stimulate mitochondrial biogenesis and enhance oxidative capacity; tailor intensity to individual tolerance.
- Sleep: Prioritize 7–9 hours of quality sleep to support cellular repair processes and optimize mitochondrial turnover through autophagy.
- Stress Management: Chronic stress increases oxidative burden; incorporate stress‑reduction practices such as meditation, breathwork, or yoga.
- Avoid Mitotoxins: Minimize exposure to substances that impair mitochondrial function, including excessive alcohol, certain medications (when possible), and environmental toxins.
Injection Technique
General subcutaneous injection guidance from clinical best‑practice resources[11][12].
- Site Preparation: Clean the vial stopper and injection site with alcohol swabs; allow to air‑dry completely (30–60 seconds) before proceeding[13].
- Drawing the Dose: Use a new sterile insulin syringe for each injection; draw the prescribed volume from the refrigerated vial; remove any visible air bubbles by gently tapping the syringe.
- Injection Angle: Pinch a fold of skin (approximately 1–2 inches); insert the needle at 45–90° depending on subcutaneous tissue thickness (90° for most adults; 45° if very lean)[11][12].
- Administration: Do not aspirate for subcutaneous injections[11]; inject slowly and steadily over 3–5 seconds; wait 5–10 seconds before withdrawing the needle to minimize leakage.
- Site Rotation: Rotate systematically between injection sites (abdomen at least 2 inches from navel, outer thighs, upper arms, upper buttocks) to prevent lipohypertrophy and local irritation[14].
- Post‑Injection: Apply gentle pressure with a clean alcohol swab or cotton ball for a few seconds; do not massage the site vigorously. A small bandage may be applied if needed.
- Disposal: Immediately place used syringes in a designated sharps container; never recap needles or dispose of them in regular trash.
Recommended Source
We recommend Pure Lab Peptides for high‑purity SS‑31 (10 mg vials).
Why Pure Lab Peptides?
- High‑purity, third‑party‑tested peptides with batch‑specific Certificates of Analysis (COAs).
- Consistent manufacturing processes aligned with quality standards and documented handling procedures.
- Reliable fulfillment and cold‑chain logistics to maintain peptide integrity during shipping.
- Transparent product information and responsive customer support for research applications.
Important Note
This content is intended for therapeutic educational purposes only and does not constitute medical advice, diagnosis, or treatment. SS‑31 is currently FDA‑approved only for Barth syndrome under the brand name Forzinity; use for other conditions remains investigational. Always consult with qualified healthcare professionals before beginning any peptide protocol. This information is provided for research and educational purposes only.
References
-
International Journal of Molecular Sciences (MDPI)
— Elamipretide: comprehensive review of structure, mechanism of action, and therapeutic potential -
British Journal of Pharmacology (PMC)
— Cardiolipin‑binding mechanism and mitochondrial membrane stabilization by SS‑31 -
U.S. Food & Drug Administration (FDA)
— FDA grants accelerated approval to elamipretide (Forzinity) as first treatment for Barth syndrome (2025) -
Journal of the American College of Cardiology (JACC)
— PROGRESS‑HF trial: safety and tolerability of elamipretide in heart failure patients -
Neurology (AAN Journals)
— MMPOWER‑2 trial: elamipretide in primary mitochondrial myopathy -
Genetics in Medicine (Nature)
— TAZPOWER trial: efficacy and safety of elamipretide in Barth syndrome -
Biochimica et Biophysica Acta (BBA) – Bioenergetics
— Cardiolipin remodeling and cristae structure: role in mitochondrial function -
Journal of Cardiovascular Pharmacology (PMC)
— SS‑31 reduces oxidative stress and improves mitochondrial bioenergetics -
Alzheimer’s Drug Discovery Foundation
— Cognitive Vitality Report: SS‑31 (elamipretide) preclinical and clinical overview -
Orphanet Journal of Rare Diseases (PMC)
— Barth syndrome: clinical presentation, diagnosis, and emerging therapies -
Centers for Disease Control and Prevention (CDC)
— Vaccine administration: subcutaneous injection technique (angle, site selection, no aspiration) -
Johns Hopkins Arthritis Center
— Patient guide: how to give a subcutaneous injection (step‑by‑step instructions) -
NCBI Bookshelf (StatPearls)
— Best practices for injection: aseptic technique, preparation, and administration -
Pharmacologic Considerations of Subcutaneous Drug Administration (PMC)
— Site rotation, absorption kinetics, and prevention of lipohypertrophy -
Pure Lab Peptides
— SS‑31 (10 mg) product page with quality documentation and batch COAs