TB-500 + BPC-157 Stack (5 mg Vials) Dosage Protocol
Quickstart Highlights
TB-500 (Thymosin β4) is a 43-amino-acid peptide that promotes tissue repair and regeneration through enhanced angiogenesis and cellular migration[1][2]. BPC-157 (Body Protection Compound-157) is a 15-amino-acid gastric peptide studied for accelerating musculoskeletal healing by upregulating growth factor signaling[3][4]. Stacking these peptides is a common research approach for synergistic tissue repair support.
- TB-500 Reconstitution: Add 2.0 mL bacteriostatic water → 2.5 mg/mL concentration.
- BPC-157 Reconstitution: Add 2.0 mL bacteriostatic water → 2.5 mg/mL concentration.
- TB-500 Dosing: 1,250 mcg (50 units) twice weekly (Weeks 1–4), then once weekly (Weeks 5–12).
- BPC-157 Dosing: 250–500 mcg (10–20 units) daily, titrated over 12 weeks.
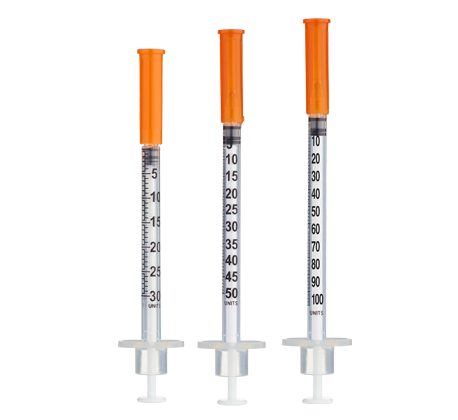
- Easy measuring: At 2.5 mg/mL, 1 unit = 0.01 mL = 25 mcg on a U-100 insulin syringe.
- Storage: Lyophilized: refrigerate at 2–8 °C (35.6–46.4 °F); after reconstitution, refrigerate and use within ~30 days.
Dosing & Reconstitution Guide
Educational guide for reconstitution and combined dosing protocol
TB-500 Protocol (2 mL Reconstitution = 2.5 mg/mL)
| Week | Dose (mcg) | Units (per injection) (mL) | Frequency |
|---|---|---|---|
| Weeks 1–4 | 1,250 mcg (1.25 mg) | 50 units (0.50 mL) | Twice weekly |
| Weeks 5–12 | 1,250 mcg (1.25 mg) | 50 units (0.50 mL) | Once weekly |
Route: Subcutaneous injection. The loading phase (Weeks 1–4) uses twice-weekly dosing to establish tissue saturation, followed by a maintenance phase (Weeks 5–12) at once weekly[1].
TB-500 Reconstitution Steps
- Draw 2.0 mL bacteriostatic water with a sterile syringe.
- Inject slowly down the vial wall; avoid foaming.
- Gently swirl/roll until dissolved (do not shake).
- Label and refrigerate at 2–8 °C (35.6–46.4 °F), protected from light.
BPC-157 Protocol (2 mL Reconstitution = 2.5 mg/mL)
| Week | Daily Dose (mcg) | Units (per injection) (mL) | Frequency |
|---|---|---|---|
| Weeks 1–2 | 250 mcg (0.25 mg) | 10 units (0.10 mL) | Once daily |
| Weeks 3–8 | 500 mcg (0.50 mg) | 20 units (0.20 mL) | Once daily |
| Weeks 9–12 | 250 mcg (0.25 mg) | 10 units (0.10 mL) | Once daily |
Route: Subcutaneous injection, preferably near the site of injury. Preclinical research commonly uses 250–500 mcg per day for musculoskeletal applications[3][4]. For ≤10-unit (≤0.10 mL) administrations, consider 30- or 50-unit insulin syringes for improved readability.
BPC-157 Reconstitution Steps
- Draw 2.0 mL bacteriostatic water with a sterile syringe.
- Inject slowly down the vial wall; avoid foaming.
- Gently swirl/roll until dissolved (do not shake).
- Label and refrigerate at 2–8 °C (35.6–46.4 °F), protected from light.
Supplies Needed
Plan based on an 8–16 week combined protocol with the schedules above.
-
TB-500 Vials (5 mg each):
- 8 weeks ≈ 3 vials
- 12 weeks ≈ 4 vials
- 16 weeks ≈ 5 vials
-
BPC-157 Vials (5 mg each):
- 8 weeks ≈ 5 vials
- 12 weeks ≈ 7 vials
- 16 weeks ≈ 8 vials
-
Insulin Syringes (U-100):
- 8 weeks: 68 syringes (TB-500: 12 + BPC-157: 56)
- 12 weeks: 100 syringes (TB-500: 16 + BPC-157: 84)
- 16 weeks: 132 syringes (TB-500: 20 + BPC-157: 112)
-
Bacteriostatic Water (10 mL bottles): Use 2.0 mL per vial for reconstitution.
- 8 weeks (8 total vials): 16 mL → 2 × 10 mL bottles
- 12 weeks (11 total vials): 22 mL → 3 × 10 mL bottles
- 16 weeks (13 total vials): 26 mL → 3 × 10 mL bottles
-
Alcohol Swabs: One for the vial stopper + one for the injection site each administration.
- 8 weeks: ~160 swabs → recommend 2 × 100-count boxes
- 12 weeks: ~232 swabs → recommend 3 × 100-count boxes
- 16 weeks: ~304 swabs → recommend 4 × 100-count boxes
Protocol Overview
Concise summary of the combined regimen.
- Goal: Support tissue repair, reduce inflammation, and accelerate recovery from musculoskeletal injuries[1][3].
- Schedule: TB-500 twice weekly (loading) then once weekly (maintenance); BPC-157 daily throughout.
- Dose Range: TB-500: 1,250 mcg per injection; BPC-157: 250–500 mcg daily.
- Reconstitution: 2.0 mL per 5 mg vial for both peptides (~2.5 mg/mL).
- Storage: Refrigerate lyophilized and reconstituted solutions; use within ~30 days after reconstitution.
Dosing Protocol
Suggested combined approach for synergistic tissue support.
- TB-500 Loading: 1,250 mcg twice weekly for 4 weeks (total ~2.5 mg/week).
- TB-500 Maintenance: 1,250 mcg once weekly for Weeks 5–12.
- BPC-157 Start: 250 mcg daily for 2 weeks, then increase to 500 mcg daily.
- BPC-157 Taper: Return to 250 mcg daily for final 4 weeks (Weeks 9–12).
- Timing: Inject at consistent times; BPC-157 preferably near injury site.
Storage Instructions
Proper storage preserves peptide quality.
- Lyophilized: Store at 2–8 °C (35.6–46.4 °F); for long-term storage, −20 °C (−4 °F) is acceptable[9].
- Reconstituted: Refrigerate at 2–8 °C (35.6–46.4 °F); use within ~30 days; avoid freeze–thaw cycles.
- Allow vials to reach room temperature before opening to reduce condensation uptake.
Important Notes
Practical considerations for consistency and safety.
- Use new sterile insulin syringes for each injection; dispose in a sharps container.
- Rotate injection sites (abdomen, thighs, upper arms) for TB-500; inject BPC-157 near the target tissue when possible[6].
- Inject slowly; wait a few seconds before withdrawing the needle.
- Document daily dose, injection site, and any observations to maintain consistency.
- Both peptides may be administered on the same day but at different injection sites.
How This Works
TB-500 mimics endogenous thymosin β4, modulating inflammation and activating repair pathways including PI3K/Akt and Notch signaling to enhance angiogenesis and cell migration[1][2]. It promotes actin sequestration and is studied for accelerating wound healing, reducing fibrosis, and improving function after ischemic or traumatic injuries.
BPC-157 upregulates angiogenic pathways (including VEGF) and counteracts inflammatory cytokines[3]. Preclinical research demonstrates improved healing of muscle, tendon, ligament, and bone with no observed toxicity[4][5]. The combination targets complementary mechanisms: TB-500 for systemic tissue repair signaling and BPC-157 for localized healing support.
Potential Benefits & Side Effects
Observations from preclinical and clinical literature.
- TB-500: Preclinical studies show accelerated wound closure, reduced fibrosis, and improved function after ischemic or traumatic injuries[1][2].
- BPC-157: Preclinical models demonstrate accelerated recovery of tendon, ligament, muscle, and bone injuries with no observed toxicity[3][4]. One clinical series found pain relief in 7 of 12 patients after a single intraarticular knee injection[4].
- Stack Rationale: The combination may offer complementary mechanisms—systemic repair signaling (TB-500) plus localized tissue healing (BPC-157).
- Side Effects: Generally well tolerated in research settings; occasional mild injection-site reactions (redness, itching) may occur with subcutaneous administration.
Lifestyle Factors
Complementary strategies for best outcomes.
- Prioritize adequate protein intake to support tissue repair and regeneration.
- Incorporate appropriate physical therapy or mobility work as tolerated during recovery.
- Prioritize sleep and stress management to optimize healing responses.
- Stay hydrated and maintain balanced nutrition to support anabolic processes.
Injection Technique
General subcutaneous guidance from clinical best-practice resources[7][8].
- Clean the vial stopper and skin with alcohol; allow to dry.
- Pinch a skinfold; insert the needle at 45–90° into subcutaneous tissue[7].
- Do not aspirate for subcutaneous injections; inject slowly and steadily[7].
- Rotate sites systematically (abdomen, thighs, upper arms) to avoid lipohypertrophy[8].
- For BPC-157, injecting near the injury site may enhance local effects[4].
Recommended Source
We recommend Pure Lab Peptides for high-purity TB-500 and BPC-157.
Why Pure Lab Peptides?
- High-purity, third-party-tested lots with batch COAs.
- Consistent, ISO-aligned handling and documentation.
- Reliable fulfillment to maintain cold-chain integrity.
Shop TB-500 at Pure Lab Peptides
Shop BPC-157 at Pure Lab Peptides
Important Note
This content is for educational purposes only and is not medical advice.
References
-
Frontiers in Endocrinology (2021)
— Progress on the Function and Application of Thymosin β4 -
Annals of the New York Academy of Sciences
— Thymosin β4: roles in development, repair, and engineering of the cardiovascular system -
Frontiers in Pharmacology (2022)
— Pharmacokinetics, distribution, metabolism, and excretion of BPC-157 -
HSS Journal (2025)
— Emerging Use of BPC-157 in Orthopaedic Sports Medicine: A Systematic Review -
Current Pharmaceutical Design (2018)
— Stable Gastric Pentadecapeptide BPC 157: Novel Therapy in Gastrointestinal Tract -
Journal of Orthopaedic Surgery and Research (2019)
— BPC 157 and its effects on tendon healing -
CDC (2024)
— Vaccine Administration: Subcutaneous (SUBCUT) Injection -
NCBI Bookshelf (2023)
— Administration of Parenteral Medications – Nursing Skills -
CDC (NCHS)
— Laboratory Procedure Manual: C-Peptide in Serum (storage guidelines) -
CDC (2023)
— General Best Practice Guidelines for Immunization: Vaccine Administration -
Pure Lab Peptides
— TB-500 (5 mg) product page -
Pure Lab Peptides
— BPC-157 (5 mg) product page